I have previously discussed my project to create a DIY flow battery using Fe/Mn chemistry. On this post I want to expand on the potential limits of this chemistry and some modifications that should enhance our ability to increase its energy density and performance.
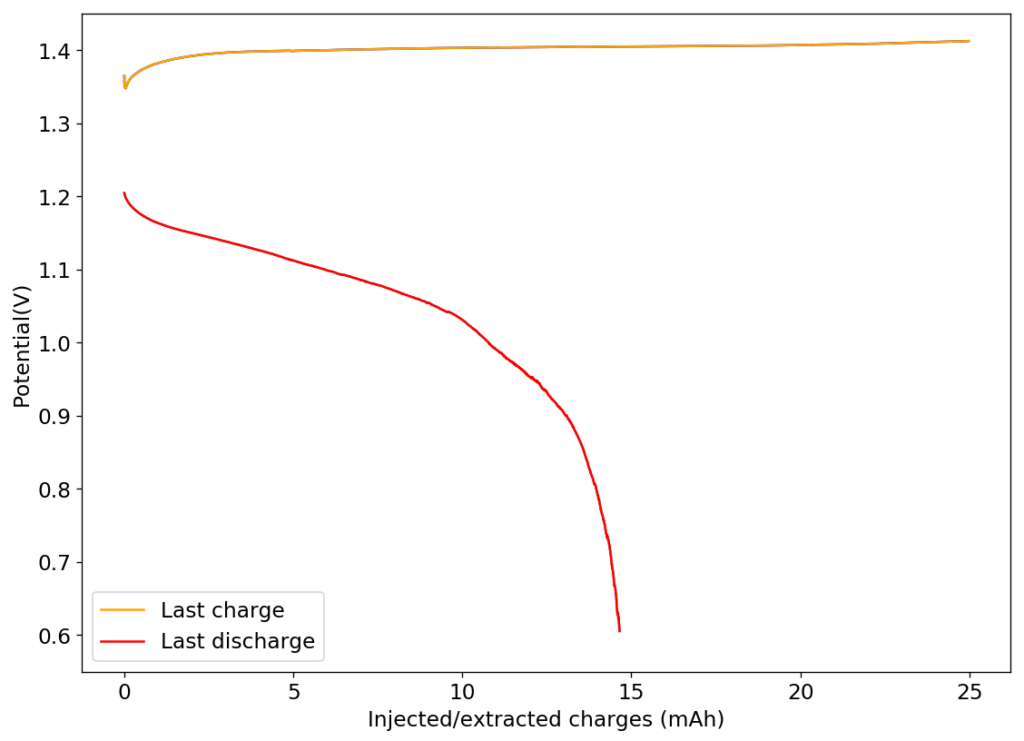
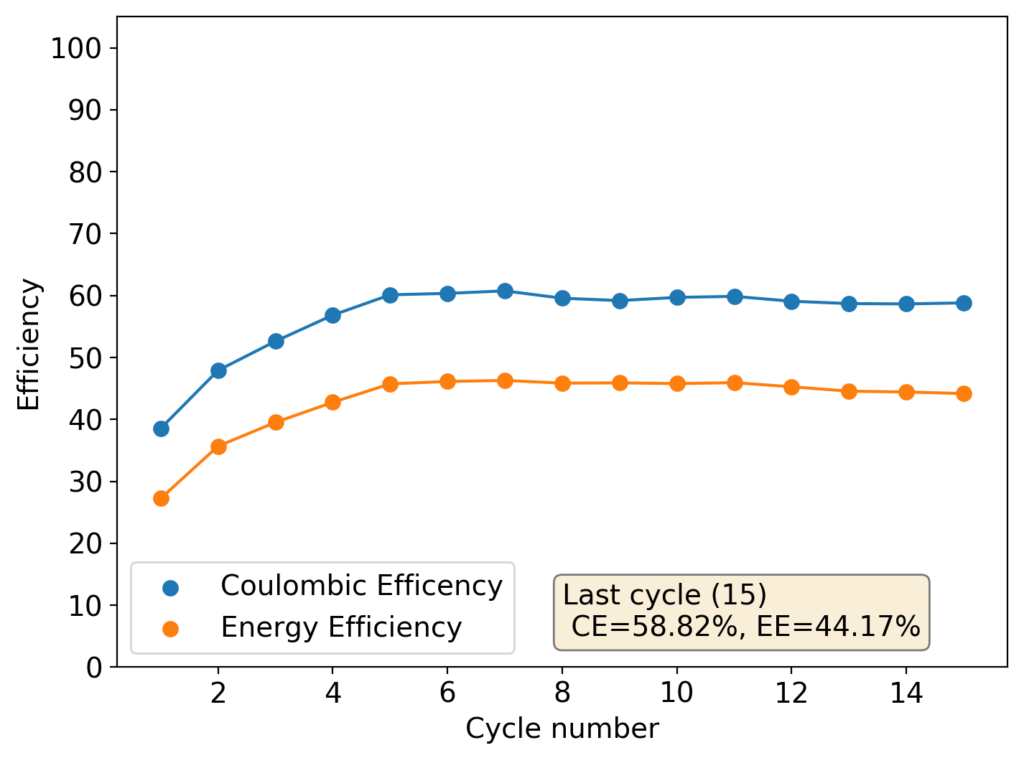
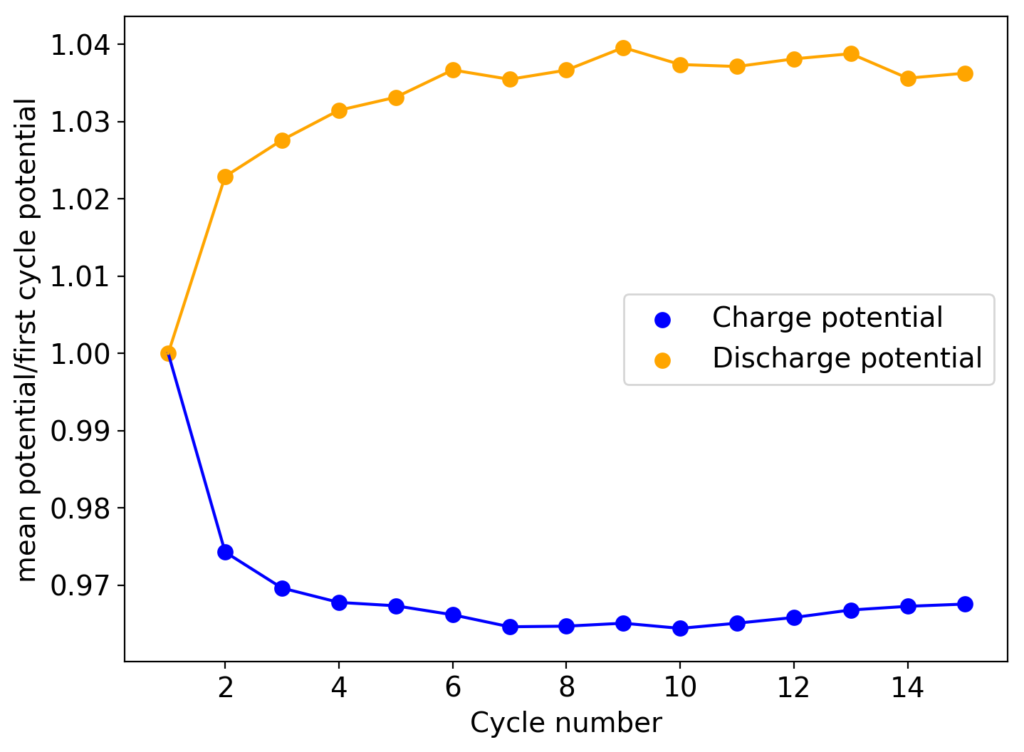
My first idea is to attempt to create a flow battery using an NaFe(EDTA) solution as anolyte and an Na2Mn(EDTA) solution as catholyte. This battery would have a potential of around 0.74V, as I measured by cyclic voltammetry (CV) of the species involved. I commented on how the limit of solubility of these chemicals – without any additives – is limited to at best around 0.5M, which limits the battery power density to around 10 Wh/L.

However, it is interesting to note that the solubility of these EDTA salts increases aggressively with pH, such that both can be dissolved above 1M at a pH of 7. I confirmed that the solubility increases aggressively as a function of pH, being able to create a solution that was around 1M for both compounds with 3M NaCl supporting electrolyte. To do this I used potassium carbonate to increase the pH gradually to the 7-7.5 range. I also confirmed that the reversibility of the electrochemistry was unaffected through CV, although both standard half-cell potentials are shifted negatively by around 50mV.
This increase in solubility is interesting, as it increases the power density of the battery substantially. If the compounds can be dissolved at 2M, then it would give the battery a density closer to lead acid, at 40Wh/L.
Sadly there are no published studies that show the solubility of EDTA salts as a function of pH, however one of the few published studies of Mn-EDTA in flow batteries (here) shows that you can dissolve Na2MnEDTA at concentrations past 1M. I have bought some additional Mn-EDTA to perform my own solubility experiments, I will let you know what I find out.
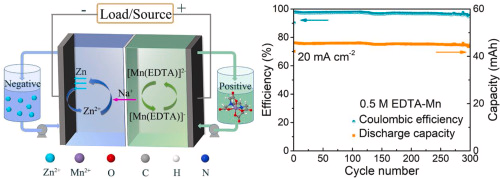
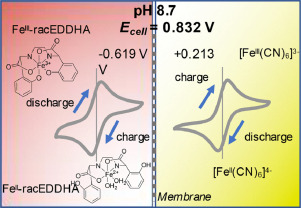
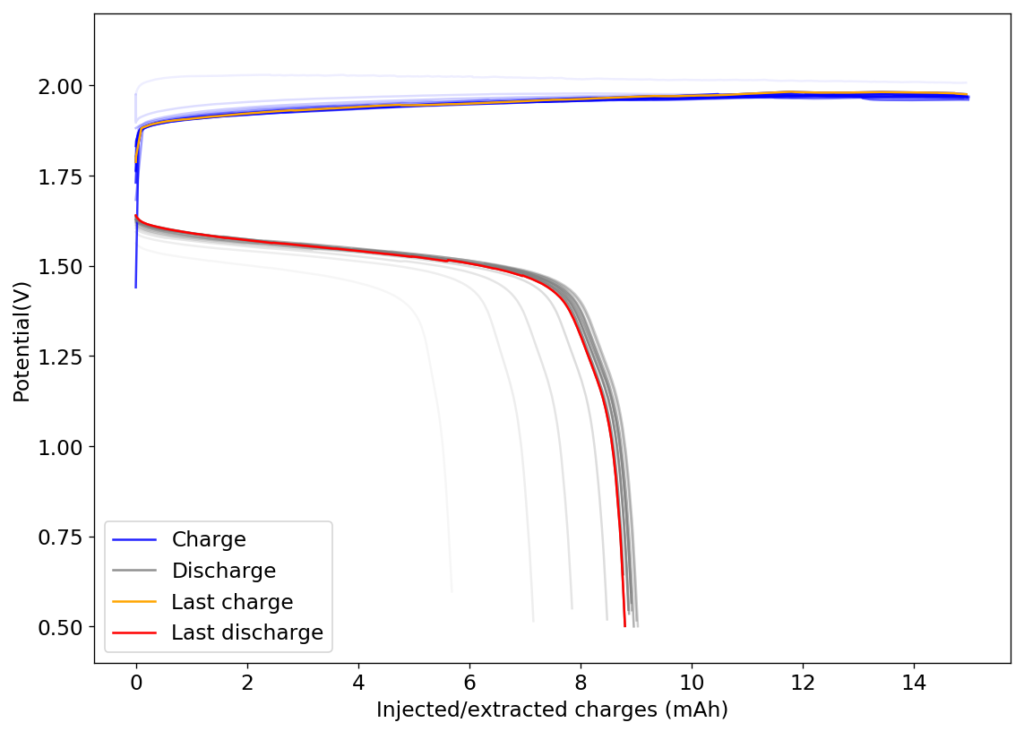
Another interesting note is to look at other Fe chelate candidates. While EDTA is the lowest cost chelate, the Fe-EDDHA chelate is interesting, as it has a significantly more negative potential Vs Ag/AgCl (-0.6V instead of -0.1V for Fe-EDTA). Recent literature of Fe-EDDHA chelate characterization and its use in flow batteries already shows its practical application (here and here). This increases the potential of an Fe/Mn battery from 0.74V to around 1.2V, which is a decent potential to achieve within the stable window of water at pH 7.
This means that, if using Fe-EDDHA, we could potentially achieve a power density of up to 80Wh/L at a solubility of 2M. If the solubility limit is around 1M, then it should still allow us to get to 40 Wh/L. With this in mind, the Fe/Mn chemistry should match lead acid power density and be a strong competitor against Vanadium based chemistries. This is especially given the fact that Fe/Mn are super abundant and this battery is based on already commercially available chemicals in water, at a neutral pH.
As you can see above, the anolyte and catholyte I propose have been tested, so this is definitely a system that can be built in a rather straightforward manner.