In order to properly research batteries at a small scale, it is necessary to have setups that have a reproducible geometry and that can be put together and taken apart many times, in the exact same way. Through all the research published in this blog, I have been using Swagelok cells to achieve this goal. In today’s post I will be talking about my Swagelok cell and how I use it to put together my batteries.

My Swagelok cell – showed above – is just a piece of PTFE pipe with an internal diameter of 0.5 inches, that has been threaded externally in order to enable the screwing of two stainless steel caps, which are the contact points that allow the connection between the internal electrodes and the external testing equipment.
Besides this external stainless steel caps, the Swagelok cell comes with some internal stainless steel electrodes, that you can use to contact the cathode and anode materials of the battery, if the chemistry allows for this. However, given the corrosive nature of elemental bromine, I use 0.5 inch graphite rods that serve as carbon electrodes. The rods have been rounded on one side – to make a nice contact with the stainless steel caps – and they are flat on the other side to fully contact the cathode and anode electrodes.
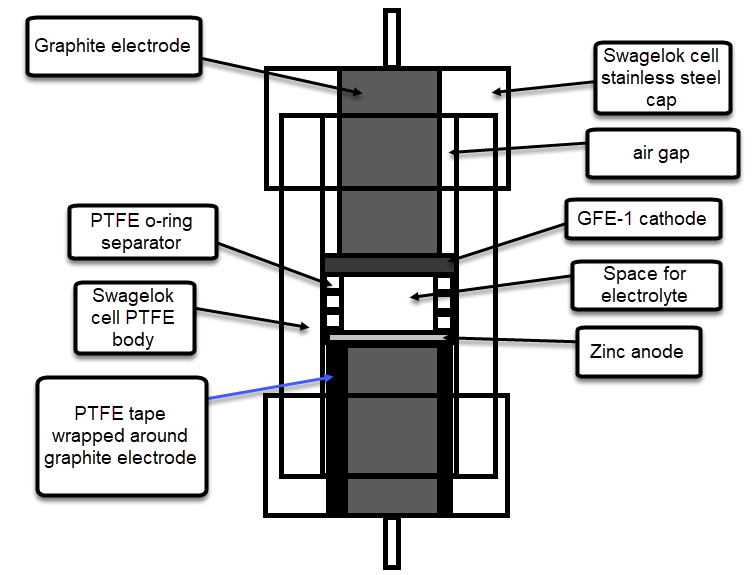
The image above shows you a diagram of a vertical cross section of the Swagelok cell when it is fully assembled with a separator-less inverted Zn-Br battery configuration. As you can see, the bottom graphite electrode is wrapped heavily in PTFE tape, in order to provide a water-tight seal, while the top electrode is only wrapped once, in order to insulate everything but the tip of the electrode from making contact with solution, allowing for an air gap to allow any excess solution to pool after compression.
As you can see, the battery comprises a small space in the middle of the Swagelok cell. The space for the electrolyte is provided by the use of PTFE o-ring spacers and when the cell is closed the space has a perfectly defined volume, since the rings do not compress. When a separator containing setup is used, these o-ring spacers are replaced by layers of non-woven fiberglass separator, which is the other material I have tried. In an inverted configuration the anode is placed at the bottom, while in a normal configuration the anode is placed on top.
Given how the battery is configured, it is easy to take it apart and put together a new battery with brand new materials to either repeat and experiment or perform an entirely new experiment with the exact same geometry. Geometry and mass are very important aspects of battery research – as they determine specific power and energy – so being able to do experiments where these two variables can be guaranteed to be as reproducible as possible is conducive to better results.
I am so impressed with your work. Exactly what I was looking for. How can I help? Can I donate funding?
Thanks for your message Robert! I appreciate your support. I will add a paypal donation link to the website in case you want to support my efforts.
Hi Daniel,
Did you saw this: https://youtu.be/j75_mYwA_E8 ? Somehow simple but it should be in depth investigated.
Best regards,
Gabriel
Hi Gabriel,
Thanks for commenting! I did watch that video. There are huge problems with the batteries that Rob proposes in that video, the internal resistance is huge – just by the interelectrode distances – so the energy efficiency of those batteries is going to be around 15% (the efficiency he refers to in the video >80% is the Coulombic efficiency, which is different and does not depend on electrical resistance). It is not viable to have a device that wastes 85% of the energy you put into it. Furthermore, the big over-potentials needed to charge these devices pretty much ensures that H2 production and the deterioration caused by it will be massive.
All-in-all, this is precisely why thorough testing of batteries with proper measuring of charge/discharge curves is needed whenever any sort of claim is made. Rob’s excuse that “these curves have been measured before by a bunch of people so I will not bother” does not cut it, because every geometry and configuration has different and often dramatically different properties. For example, if he had measured the charge/discharge curves of this jar configuration it would have been pretty obvious that this sort of device is simply not practical and – besides for demonstration purposes – a waste of battery materials and resources.
I applaud Rob’s efforts to share information and generate excitement about DIY science. But when he is actually talking about practical applications his lack of data can make the videos highly misleading, especially for those seeking to actually build these things.
Thanks again for your comment, I hope the above helps 🙂
Daniel
Hello. Can you tell me where you purchased this cell or the components?
You can buy the cells on ebay. Note that the stainless steel electrodes are not fit for Zn-Br batteries, so you’ll need to buy 1/2 inch graphite rods and cut them for the cell. I bought those on amazon here. I hope this helps!
I guess you have seen this? https://www.sciencedirect.com/science/article/pii/S2589004220305356
Yes, I have talked about this paper extensively in this blog and why it is pure smoke. In summary, the batteries only appear good because they normalize all performance to just 3mg of cathode mass, which is ridiculous. If you normalize to the actual total cathode mass they use, the performance values are 100x smaller. The TPABr is not a great complexing agent for these batteries either, it is highly insoluble in concentrated ZnBr2 solutions, which limits the maximum concentration of ZnBr2 to around 0.5M, which is just not practical for high density batteries (we need at least >2M for batteries to come even close to lead acid capacity values). Read this post for more information https://chemisting.com/2020/09/12/zinc-bromine-batteries-can-they-really-be-that-good/.
Have you seen this: https://www.nature.com/articles/s41467-020-20331-9.pdf
They are using Iodine in a particular way. They also mention a solvent, acetonitrile. Maybe there is some useful info in the paper that might help with getting a functional battery going.
Cheers.
Thanks for your comment! I would encourage you to read my posts on non-aqueous Zn-Br batteries. Note that acetonitrile is a very toxic solvent, so there’s no way I’m using that in my house.
Pingback: Towards a practical, high efficiency, high capacity DIY Zinc-Iodine battery | Chemisting