I’ve done a lot of experiments during the last two years around the construction of Zinc halide batteries, in particular, Zn-Br and Zn-I batteries. So far, I have been unable to find a battery setup that provides high energy and coulombic efficiencies, high capacity and high stability.
Zinc-Bromine batteries suffers from problems related with Br2 diffusion, zinc dendrites, hydrogen evolution and other problems that arise when you try to resolve these issues. Trying to add complexing agents, increase electrode distance or add physical barriers, will often increase coulombic efficiency at the expense of energy efficiency, such that batteries with low self-discharge or high capacity will tend to have extremely low energy efficiency values (often below 20%). Having a battery where you need to put 20kWh in to take 2kWh out is just not a viable strategy for most people. Hydrogen evolution reactions are also inevitable with Zn-Br batteries, and these irreversibly destroy the electrolyte as a function of time.
Zn-Br batteries might be easy to show in online videos and tout as a great chemistry with lots of possibilities, but the reality of constructing a >60Wh/L static Zn-Br battery with a long cycle life shows that this is extremely hard to achieve. This is why you will find no one showing proper testing – charge/discharge curves with CE and EE numbers for many cycles – of such DIY static batteries. You can read other posts in my blog and this forum thread, for more information about my journey in Zn-Br batteries.
The above were the reasons why – after doing a lot of experiments to get to know these batteries quantitatively – I decided to move to the Zinc-Iodine chemistry.
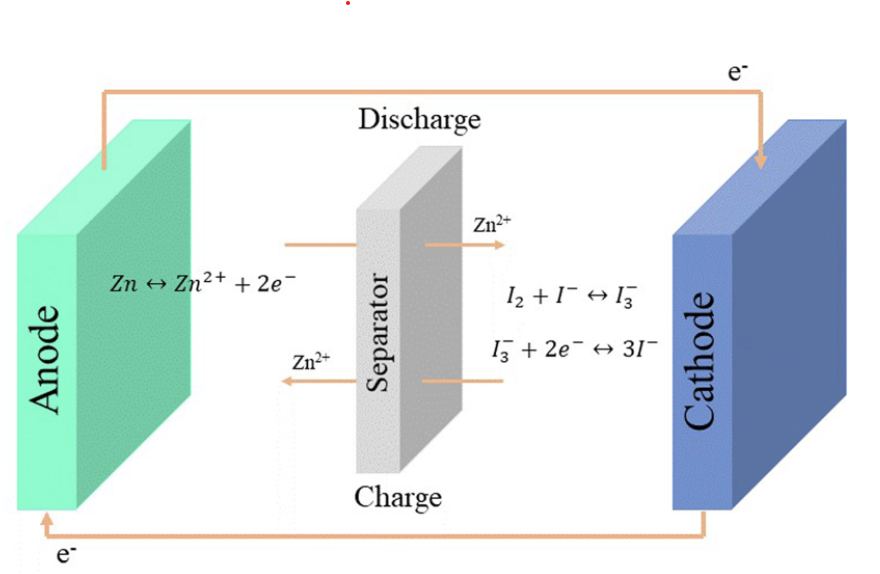
Zinc-Iodine batteries do not suffer from hydrogen evolution issues – due to the lower potential needed to charge the battery – but they also have strong problems dealing with I2 migration, especially due to the very iodide rich electrolyte, which generated a lot of readily soluble triiodide (I3–). Although many solutions to these problems have been tried and published in peer reviewed journals, most are extremely hard to apply in practice and out of reach for someone with limited equipment and resources.
However, there is some hope. A paper published in 2019 got around the problem of the triiodide ion by using a “water in salt” approach (WiS). That is, they create a very highly concentrated solution of zinc chloride and potassium iodide, where water is a very minor component by mass. In this case, they add around 2g of ZnCl2 and 0.8g of KI to only 1mL of distilled water. In such a concentrated salt solution, iodide prefers to bond to Zn to form complexes, as the formation of the triiodide ions is strongly disfavored by the environment. This is proven extensively in the paper by the use of Raman spectroscopy, supported by computational chemistry calculations.
Thankfully, both ZnCl2 and KI are readily available chemicals plus, the electrode and separator material used by the researchers are also easy to get. The cathode is made out of carbon paper, the anode is zinc metal and the separator is cellulose filter paper. I purchased the salts for around 4 USD/kg (ZnCl2) and 40 USD/kg (KI).
Note that ZnCl2 is extremely hygroscopic, so it needs to be kept under airtight conditions. Solutions of ZnCl2 are also extremely acidic (pH < 1) so you need to be very careful when handling ZnCl2 solutions that are this concentrated. Zinc chloride also heats solutions aggressively when dissolved.
The results of trying to reproduce their research were quite astonishing. I prepared the electrolyte as published and used my Swagelok cell for testing. I used a Whatman 42 filter paper as separator and an HCB-1071 carbon cloth as cathode (note the variety kit has the 15mil, which is the one I used). For the anode, I used the surface of the graphite electrode of my Swagelok cell. In total, the thickness of my battery was just 350 microns (0.0350cm).
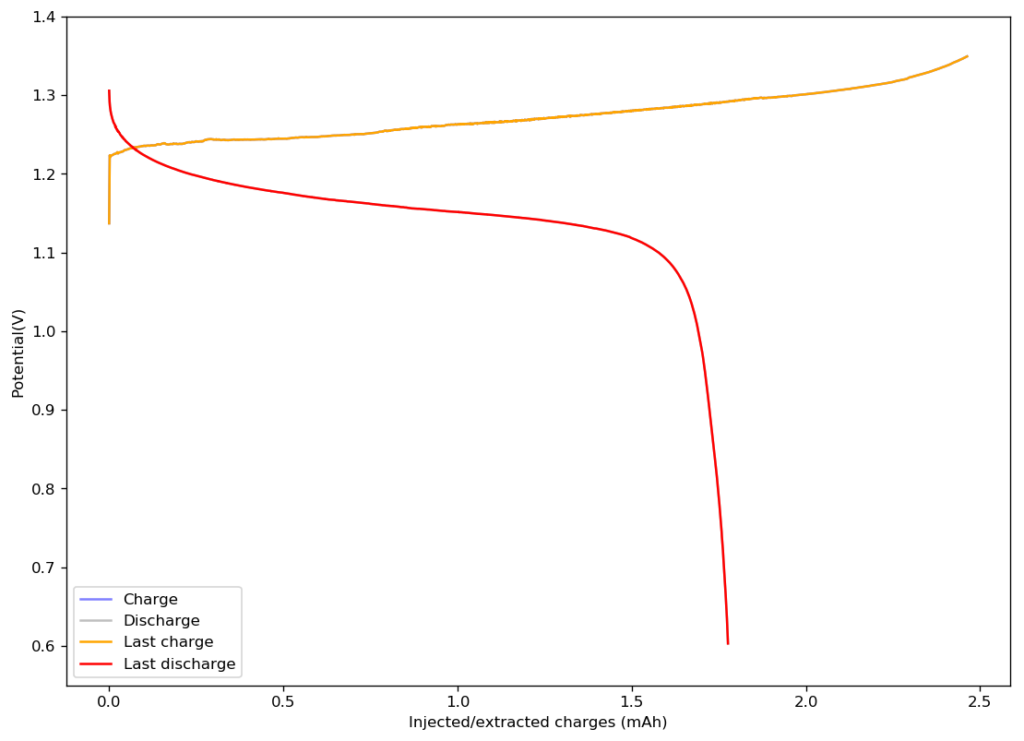
The above image shows you the results from the first charge/discharge cycle of this device. The capacity in this first cycle was 1.77mAh. Given the volume of this battery, the energy density is currently at 58Wh/L, the best energy density I have been able to create for any device.
I am now going to study the cycling characteristics of these devices further. I want to determine how the CE and EE change as a function of time and how the capacity changes as well. Taking this device apart confirms that elemental iodine is deposited on the carbon cloth and no appreciable triiodide – which has an orange color – is formed. This is also confirmed by the massive jump in CE and EE in these devices.
The above chemistry finally points to a path for a DIY battery that can be easy to build with readily available chemicals and materials. My hope is that this can lead to practical capacities in the >50Wh/L, with reasonable costs and hopefully low self-discharge and a long cycle life.
Good to see you are getting positive results, and I’m looking forward to seeing your cycle test results 🙂
Unfortunately KI is very expensive in Australia so I don’t think I’ll be testing a ZnCl2/KI battery any time soon. I’ll keep looking abroad for a better deal.
I do have Sodium Iodide. Do you think there would be any chance of a ZnCl2/NaI battery working (to some degree)?
Thanks for commenting Giancarlo! Sodium iodide should be able to work as well, although I don’t know how different the performance will be. To have the same concentration you would need to add 2.04g of ZnCl2 and 0.75g of NaI to 1mL of water.
I ran some numbers on the batteries to see just what it would take in cost and to compare to other things already done. Now this may bore you to death and I may have gotten some numbers wrong but if they are not wrong then it may be that the ZnCl battery is not the best path to follow and something else might be more profitably done with the time you spend on ZnCl batteries. And yes it’s long but I wanted show my work and show how I came to some conclusions and maybe add some ideas on ways to accomplish the same DIY battery tech but cheaper.
ZnCl2
Density 2.907 g/cm3
2g of ZnCl2/2.907 g/cm3=0.687994 cm^3
KI
Density 3.13 g/cm3
0.8g of KI/3.13 g/cm3=0.25559105 cm^3
plus 1 cm^3 water
(this below may be completely wrong because I have no idea of the density of all these added components and adding them together singly is probably not right, but it’s the best I can do and it’s close enough to get some small cost estimates)
Density of 1 unit of formula
0.687994 ZnCl2 cm^3+0.25559105 KI cm^3+1 H2O cm^3=1.94358556 cm^3
Units of formula to make a liter
1000 cm^3/1.94358556 cm^3=514.512983 calling this units of formula to make a liter
So units of formula times grams in formula to separate components
2g of ZnCl2*514.512983=1029.0 g
0.8g of KI*514.512983=411.6 g
1 cm^3 water*514.512983=514.5 g
cost
I purchased the salts for around 4 USD/kg (ZnCl2) and 40 USD/kg (KI)
I purchased the salts for around .004 USD/g (ZnCl2) and .040 USD/g (KI)
1029.0g ZnCl2 * .004 USD=4.11
411.6g KI * .040 USD=16.46 (this one seems to be the one that’s killing the price)
4.11+16.46=$20.58
So total grams equals 1954.6 g or using 58Wh/L and 1954.6 g it equals, 29.68 Wh/Kg
$20.58/58Wh=$0.35 per Wh
Not as good as I originally thought. If it didn’t go bad constantly like lead acid then it would still be better by far.
comparing it to lead-acid(I got these numbers from a table)
$0.17 per Wh
41 Wh/kg
100 Wh/liter
Some cost I’ve seen for lithium cells in cars
Tesla’s battery packs cost, on average
$187 per kWh
GM’s packs cost
$207 per kWh
auto industry spends an average
$246 per kWh for battery packs.
So $18,000 for Teslas 100KWh pack in it’s car is big bucks. Let’s look at ZnCl battery at material cost, without separators, $20.58 for 58Wh/L
So 100KWh/58Wh=1724.1379 batches of 1 liter to make 100KWh
and each batch cost for the 58Wh cost $20.58, so to cost out 100KWh,
1724.1379 * $20.58=$35,482.75, not good. Maybe I made an error somewhere. I looked at it a couple of times and didn’t see it if I did.
I was reading about Ferrocene-based flow batteries the other day and they were talking about $66/kWh which is great. So $0.06 per Wh. Really, really cheap. Of course I don’t have total numbers in what you would need to build a cell with large current or voltage. May be a different story couuntig the whole package.
A Durable, Inexpensive and Scalable Redox Flow Battery Based on Iron Sulfate and Anthraquinone Disulfonic Acid
https://iopscience.iop.org/article/10.1149/1945-7111/ab84f8
Could you use activated carbon for electrodes? The reason I’m so interested in this is it’s something I could DIY cheap. I read about a company that DARPA funded that could taylor carbon foams to different pore size and different conductivities but they don’t publish so…
If I’m not mistaken activated carbon can be made with a pine board that is stuck in a closed metal can with a small hole in the bottom and then fired so that all the wood gas is burnt off and what you are left with is just the carbon and maybe a few minerals in the board. Another possible activated carbon electrode could be made the same way but with only plain unbleached paper towels fired the same way. I’ve been looking at research papers to try and get a handle on how to make low cost carbon electrodes.
I’ve been collected papers on this the last few days, Mostly for capacitors that are also batteries. I think. Robert Murray Smith talks about this and it is a lot of what he is aiming for.
Here’s a set of papers on carbon
https://www.frontiersin.org/research-topics/4162/carbon-materials-for-sustainable-and-affordable-low-carbon-energy-technologies#articles
More papers and no I have not read them all yet. If you’re interested in this sort of thing you can find the papers here
http://libgen.rs/
click on the button for scientific articles under the search space then search
Fabrication and Characterization of Electrochemical Double Layer Capacitor Using Biomass Based Activated Carbon Electrode2013[ECS Meeting Abstracts ].pdf
Fabrication of a carbon electrode using activated carbon powder and application to the capacitive deionization process(2010)[Separation and Purification Technology vol. 70 iss. 3] Jae-Hwani.pdf
Fabrication of supercapacitor using banyan leaves-based activated carbon electrode and formic acid-based polymer electrolyte2020[Materials Today_ Proceedings ]Tripathi, Mukta.pdf
Flexible Type Symmetric Supercapacitor Electrode Fabrication Using Phosphoric Acid-Activated Carbon Nanomaterials Derived from Cow Dung for Ren, Energy App,2020[ACS Omega vol. 5 iss. 25] Rajabathar.pdf
High-energy-density hybrid electrochemical capacitor using graphitizable carbon activated with KOH for positive electrode2007[Journal of Power Sources vol. 166 iss. 2] Taira Aidapd.pdf
Preparation of Nano-Porous Activated Carbon Aerogel Using a Single-Step Activation Method for Use as High-Power EDLC Electrode in Organic Electrolyte2016[Jou.of Nanosc.e and Nanotech. vol. 16 iss. 5] Kwon, Soo.pdf
The fascinating supercapacitive performance of activated carbon electrodes with enhanced energy density in multifarious electrolytes supplementryc9se01298b1.pdf
The fascinating supercapacitive performance of activated carbon electrodes with enhanced energy density in multifarious electrolytes(2020[Sustainable Energy Fuels ] M, Karnan.pdf
Robert Murray Smith talks about it and think some of these are about capacitors that are also batteries. He a little while ago talked about what I remember as Liposomes. I know what this is because I make liposomal vitamin C in a ultrasonic mixer. The significance of this is for some reason, not entirely clear, the liposomes boost the capacitance by about a million times. Wow. Now I know what you said about Murry and I don’t disagree but none the less he has made some of these and he has very good test equipment to test this stuffs capacity and the liposome stiff works out. It’s a far cry from something you can put in your car but it’s just as good a start as any other. What’s important is that the material be readily available and low cost DIY.
Oops. My apologies about asking about weird carbon electrodes. I asked you about this before but forgot and only realized it after I commented.
Hi
This is Rouzbeh, I am an entrepreneur from Iran who’s been working on battery technology especially zinc batteries. Recently, I found your blog. It is awesome. Thanks for sharing your experiments.
I would be very grateful if you send me your email address, so I can share some of my experiments and problems with you. Maybe you could help me with them.
Hope to hear from you.
best regards,
Rouzbeh
Hi there,
I’m Bret, I’m trying to make a good zinc based battery as well. I’m trying to figure out a way to have a good capacity with low self discharge.
It seems the only way is to remove the zinc or the fluid to allow storage of charge. Then we get a redox flow battery?
Any advance on your batteries?