In order to properly research batteries at a small scale, it is necessary to have setups that have a reproducible geometry and that can be put together and taken apart many times, in the exact same way. Through all the research published in this blog, I have been using Swagelok cells to achieve this goal. In today’s post I will be talking about my Swagelok cell and how I use it to put together my batteries.

My Swagelok cell – showed above – is just a piece of PTFE pipe with an internal diameter of 0.5 inches, that has been threaded externally in order to enable the screwing of two stainless steel caps, which are the contact points that allow the connection between the internal electrodes and the external testing equipment.
Besides this external stainless steel caps, the Swagelok cell comes with some internal stainless steel electrodes, that you can use to contact the cathode and anode materials of the battery, if the chemistry allows for this. However, given the corrosive nature of elemental bromine, I use 0.5 inch graphite rods that serve as carbon electrodes. The rods have been rounded on one side – to make a nice contact with the stainless steel caps – and they are flat on the other side to fully contact the cathode and anode electrodes.
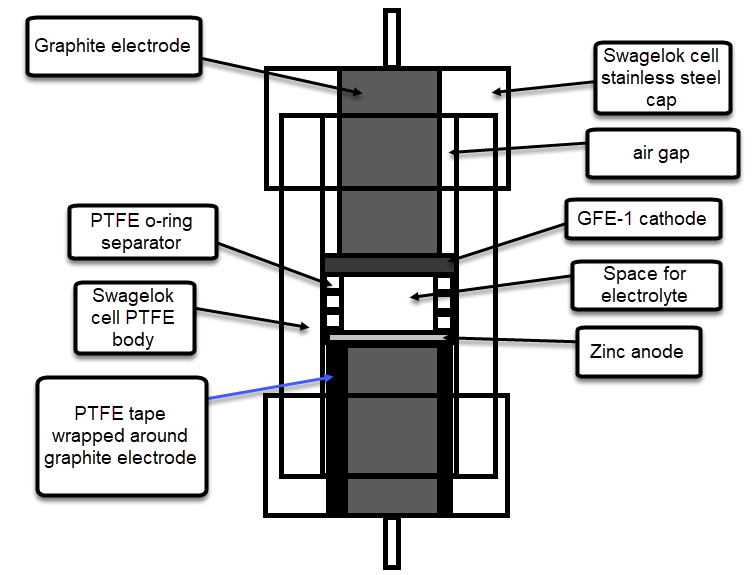
The image above shows you a diagram of a vertical cross section of the Swagelok cell when it is fully assembled with a separator-less inverted Zn-Br battery configuration. As you can see, the bottom graphite electrode is wrapped heavily in PTFE tape, in order to provide a water-tight seal, while the top electrode is only wrapped once, in order to insulate everything but the tip of the electrode from making contact with solution, allowing for an air gap to allow any excess solution to pool after compression.
As you can see, the battery comprises a small space in the middle of the Swagelok cell. The space for the electrolyte is provided by the use of PTFE o-ring spacers and when the cell is closed the space has a perfectly defined volume, since the rings do not compress. When a separator containing setup is used, these o-ring spacers are replaced by layers of non-woven fiberglass separator, which is the other material I have tried. In an inverted configuration the anode is placed at the bottom, while in a normal configuration the anode is placed on top.
Given how the battery is configured, it is easy to take it apart and put together a new battery with brand new materials to either repeat and experiment or perform an entirely new experiment with the exact same geometry. Geometry and mass are very important aspects of battery research – as they determine specific power and energy – so being able to do experiments where these two variables can be guaranteed to be as reproducible as possible is conducive to better results.