On my last post, I showed the results of charging/discharging a flow battery using a ZnCl2+NH4Cl+KI electrolyte using 4 layers of Daramic as a membrane. However, while Daramic is a low cost material, it is not easily accessible for DIY testing at this moment. For this reason, I wanted to run some tests on materials that are easier to source than Daramic.
I looked for materials around my house that had similar porosity (0.1-5um). I tested several different papers that I had around but none of them worked very well. The porosity of most traditional printer papers is high, with most having 10-20um pore sizes. This means that you need many layers to prevent fast self-discharge from migration of the triiodide across the membrane. Additionally, the papers lost structural integrity quite easily.
Finally, I stumbled upon matte photo paper as a potential solution. This paper has much lower porosity with <5um pore sizes. Some of these papers might even have pore sizes that are below 1um. This is important for printing photographs, as low pore sizes implies that there is less bleeding of ink when it is applied to the paper, although ink needs to be applied much more slowly to the material (reason why printing with these papers is really slow).
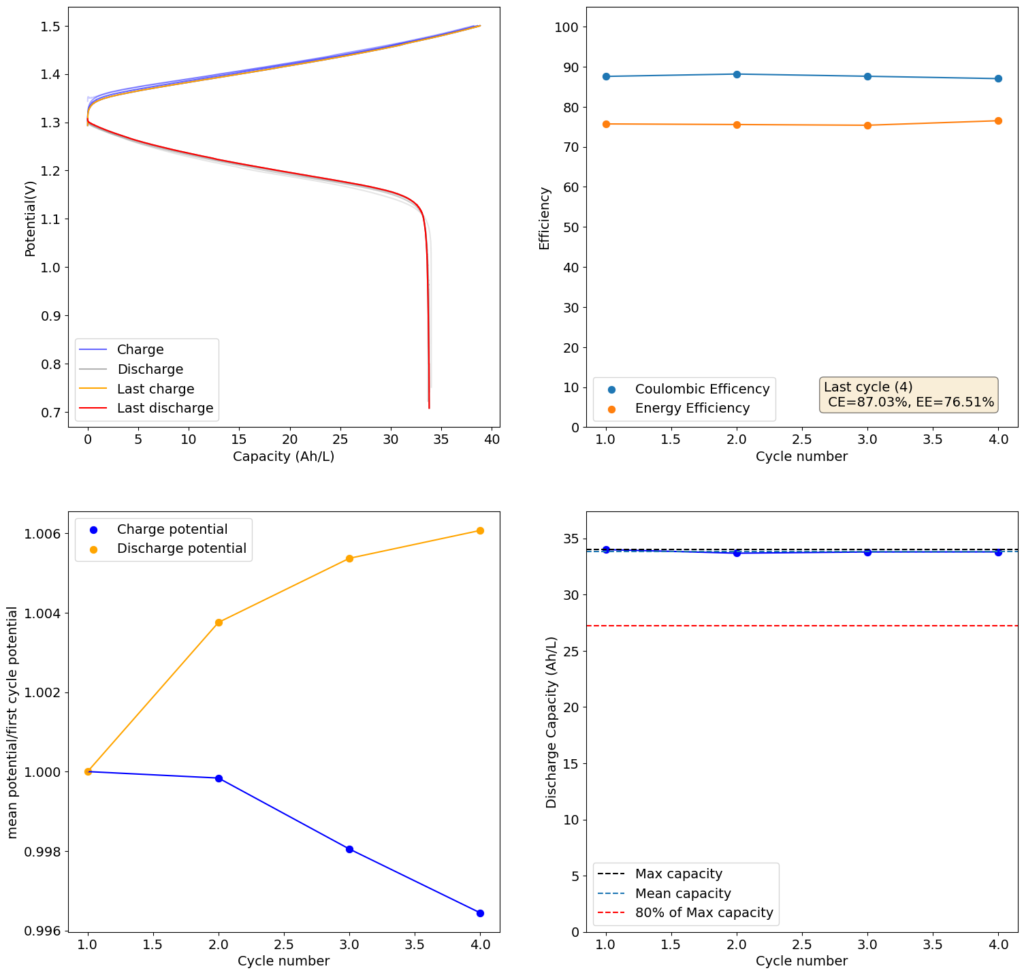
For my initial test, I used 4 layers of matte photo paper. The paper does have a substantially higher ohmic resistance compared to Daramic, so I had to lower the current density to 20mA/cm2. I did 4 cycles of charge/discharge that you can see above (I only did 4 because the lower current meant cycling was quite slow). The CE of 87.54% and EE of 75.72% with a capacity of 33.8 Ah/L shows that photo paper is definitely a good choice for at least the short term cycling of these devices.
On inspection, the photo paper did not show any evident degradation although dendrite penetration happened just as much as it did with the Daramic separator. The separator was also completely black, fully permeated by the catholyte solution which contains triiodide in solution when charged.
Hello.
I have been doing my own layman style research into emerging battery technologies that may allow me to DIY a battery that could possibly be used to power a boat.
Redox flow batteries seem fairly easy to build, and I was wondering if you might be able to recommend some chemistry to use.
Ideally, I am looking for chemistries that are non-toxic/non-hazardous/cheap and allow for batteries that are at least able to function reliably over time (energy density is great, but not my highest priority).
Would you happen to have some recommendations in that regard?
Thanks
So far the best candidate I have found is the Zinc-Iodide chemistry detailed in this post. Feel free to ask any questions about it you might have.
The work that you do as independent chemist is amazing and the fact that you are willing to help average laymen makes it that much greater. I am currently focused on finishing up a construction project as well as looking for my next role as software developer. Once I have a studio set up in my new home then maybe we’ll be able to casually collaborate on something.
Thanks for your support and kind words!
Hi Daniel,
Your blog is awesome. I have been trying to build a DIY with the same goal as you. Flow batteries seem quite complicated to build so I have been playing with non-flow ZnBr2 batteries. I am currently on version 8 of my cell design, which is a ZNBr2 gel using graphite foil as current collector. I have been following Robert Murray’s work and building from that. My problem is that I don’t know when to tell if my cell is good enough for me to scale it to the next level or if I need more changes.
I would appreciate any help that you or anybody else can give me. If you are interested I can send you a link to my YouTube channel where I am recording my progress. They are super bad amateur videos but I’m doing it to share information not to make money from YouTube.
Thanks
Grant
Thanks for sharing Grant. The main priority is to keep the scale small until you have it very well characterized. If things don’t work at a small scale, they definitely wont work at a bigger scale. The small scale should be tiny, probably an area of around 1-2cm^2, this makes the production of any harmful chemicals small, allows you to do many tests with very little loss of material and allows you to cycle quickly. Rather than trying to build larger batteries, build batteries that are well understood. Prioritize characterization, get a potentiostat, measure your coulomb and energy efficiencies, your cycling stability, etc. Do not scale a battery until you have measured its CE, EE and stored charge stability for >1000 cycles.
Feel free to share your youtube channel here, I would be happy to give it a watch. Let me know if you have any further questions.
Hi Daniel,
Thanks for this. Currently I am trying to reach 50 cycles, it takes forever to run one cycle test with my current cell design, I will have to scale down the size dramatically to do what you are suggesting.
Can a potentiostat / testing device be automated to cycle my battery and record the results? Everything is manual at the moment on my side so it is very slow and painful process. You can check out my progress here https://www.youtube.com/@OpenBatteryFoundation
Thanks for your reply and sharing your channel! Yes, you can fully automate the cycling using a potentiostat like this one (https://www.pcbway.com/project/shareproject/MyStat_Potentiostat_9df57df2.html). For your battery tests get a Swagelok cell (like this one https://www.alibaba.com/product-detail/Swagelok-cell-Swagelok-battery-split-test_1600275096333.html). You can then assemble cells that are tiny, very fast to cycle and little material use.
Just wanted to stop in and give some encouragement. I was scrolling through some of the easy to reproduce flow battery research and happened to find this.
Thanks for sharing!
Hello, I recently found your blog while research at-home / DIY flow batteries. I’m very interested in this zinc iodine redox pair. I stumbled on this paper which details a iodine- carbon cathode that boasts high energy density. Have you considered trying a specialized cathode such as this? https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10484974/#CR
Also, I found your post about the Naflon equivalent membranes. Considering it is a cation exchange membrane, could this work to prevent tri and poly iodides from causing havoc after many cycles?
I’m following with great interest and great appreciation for your dedication and continual efforts.