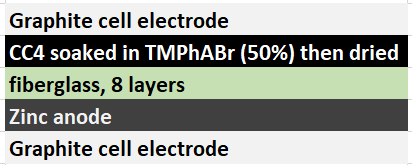
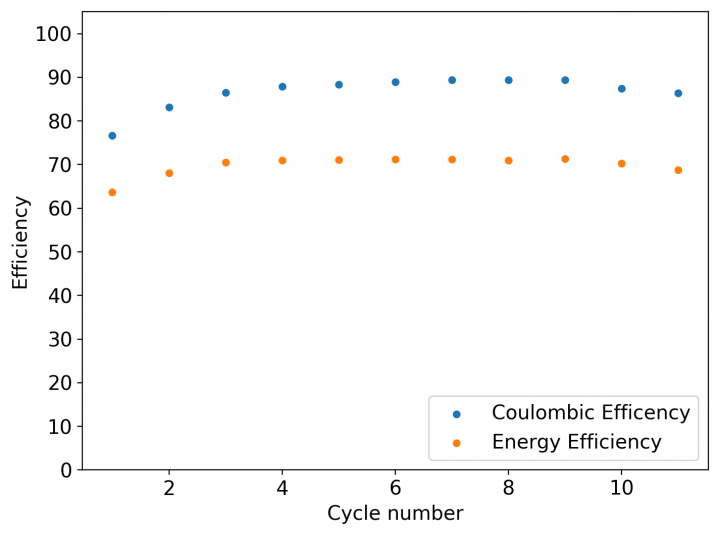
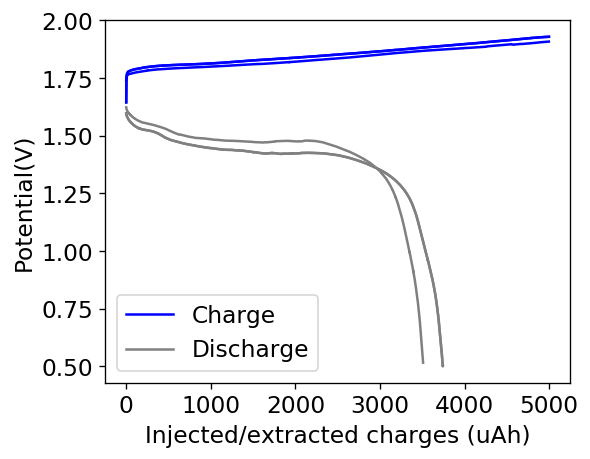
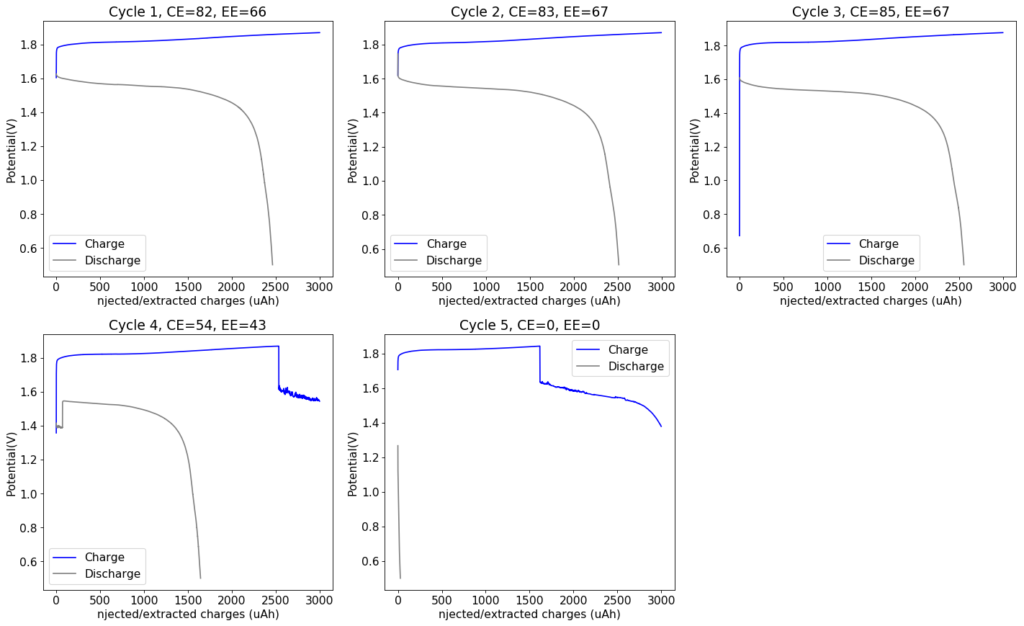
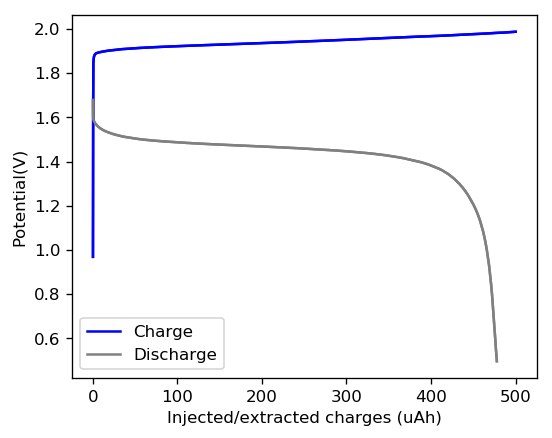
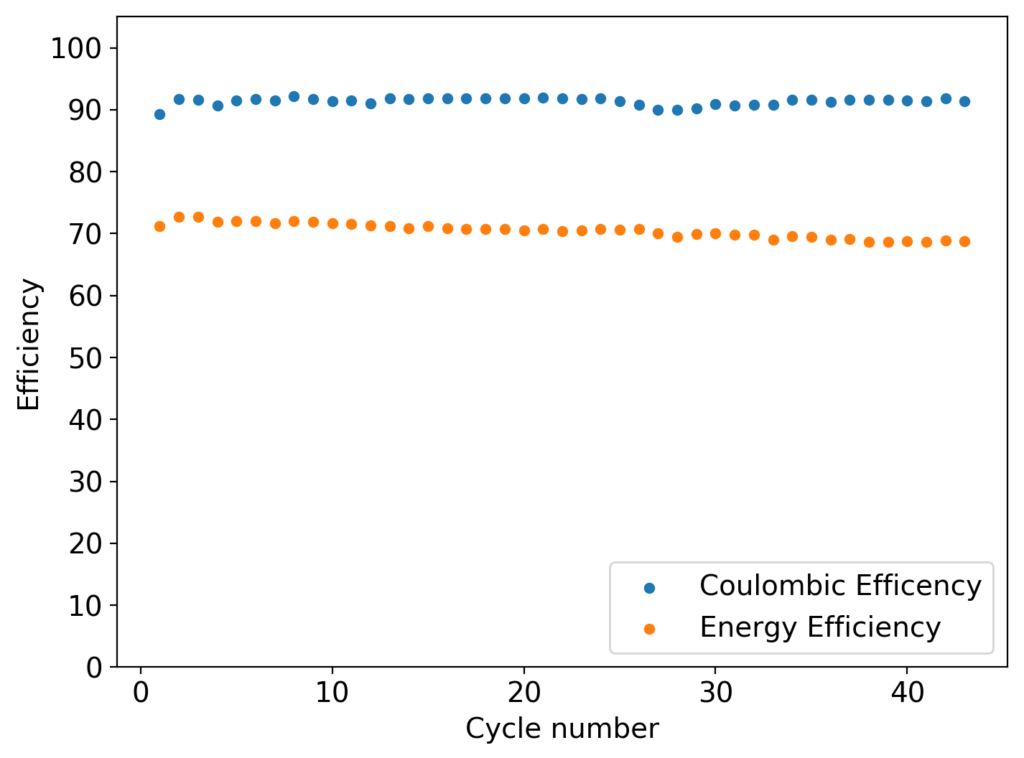
My experiments using carbon cloth cathodes have helped me construct some decent static Zinc-Bromine batteries. In particular, the CC4 carbon cathodes have been very flexible and have been used throughout most of my experiments. The last experiment I did, with a CC4 cathode previously soaked in a 50% solution of TMPhABr solution and then air dried, have shown a CE=91% with an EE=70% at a charge/discharge current of 5mA, charging to 3000 uAh and discharging to 0.5V.

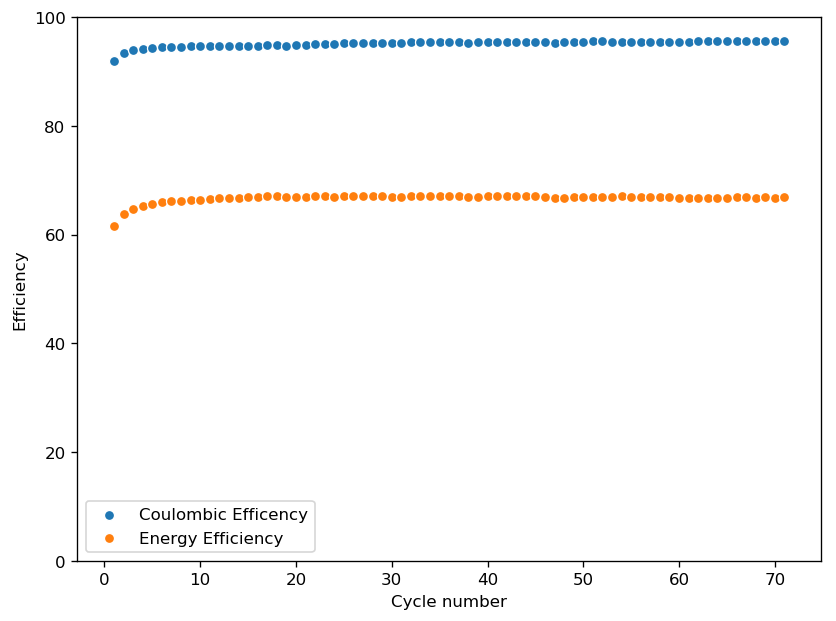
Coupling these cathodes with a ZnBr2 3M solution with 10% PEG200 has allowed me to achieve specific power values in the region of 30-40 Wh/kg – total weight of cell – with more than 40 charge/discharge cycles (see above), without the formation of any Zinc dendrites (which would short batteries after only 10-20 cycles at this charge density in previous battery tests). Higher PEG200 concentrations cause significant increases in the internal resistance of the cell while lower concentrations (<5%) are just not effective at preventing Zinc dendrite formation when using metallic Zinc anodes.
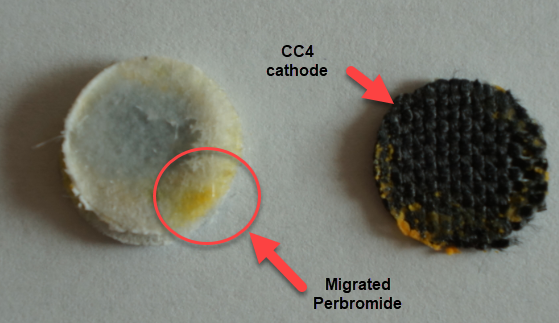
Despite the good results, I have yet to achieve high energy efficiencies, mainly due to a couple of problems. The first is that significant bromine diffusion is happening due to a lot of bromine being formed at the surface of the CC4 cathode without enough presence of TMPhABr to capture it and the second, that the internal resistance of the cell was still significantly high, owing to the significant resistance of the CC4 cathode being used.
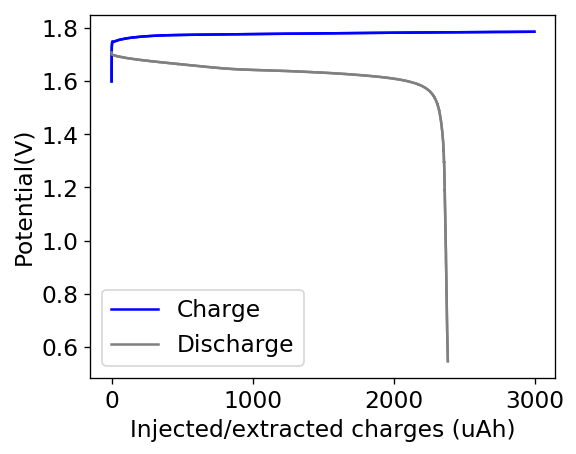
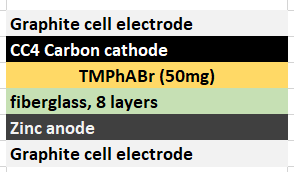
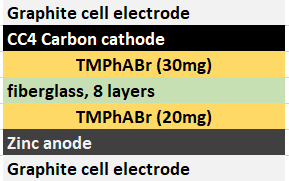
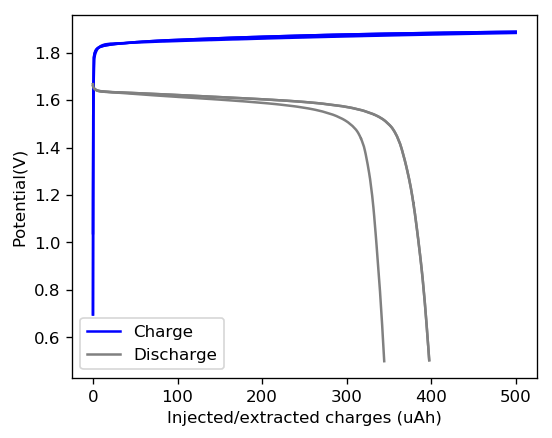
In order to attempt to solve these problems, I have decided to change to a carbon cathode that is both significantly more conductive and possesses a significantly higher surface area compared to CC4. My choice material being the GFE-1 carbon felt. For the first test I have soaked a piece of cathode in 10% TMPhABr and air-dried it before use.
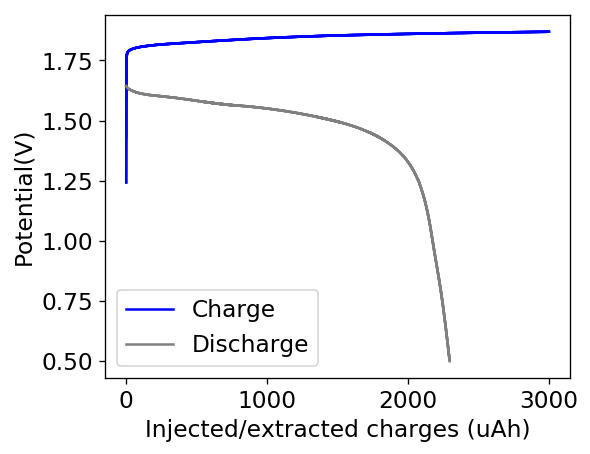
You can see the first charge/discharge curve ever produced in this configuration above. The charging potential is already significantly lower than that of the CC4 electrode and the discharge potential significantly higher, both signs of a markedly lower internal resistance. For this first cycle the Coulombic efficiency was 79% while the energy efficiency was 72%. We’ll see if the CE and EE of this battery improves as its cycled and whether or not this cathode leads to more stable cycling than the CC4 cloth electrodes!