As I mentioned in a previous post, the most important issue with the use of tetrabutylammoniumbromide (TBABr) in static Zn-Br batteries, is that the solubility of TBABr drops very sharply when zinc bromide is also in solution. While you can prepare 50% w/w solutions of TBABr in distilled water, the max concentration drops to around 0.15M when preparing solutions in the presence of 0.5M of zinc bromide. This is very bad because – in order to function as an effective sequestering agent – we would want the concentration of TBABr to be able to be significantly higher in solution.

The solubility of TBABr drops because of a sharp increase in the polarity of the solution due to the introduction of the Zn+2 ions, which are small and – due to their double charge – substantially increase the dielectric constant of the medium. The TBA+ cation is actually not that polar, being spherical and with a strong aliphatic component, meaning it cannot very successfully interact with this new, much more polar medium. As a consequence the TBABr drops out of solution.
In order to prevent this from happening, we need to find solutions that either make the Zn cation less polar or make the media less polar by introducing a less polar additive that can compensate for the increase in polarity brought by the Zn cation. These two potential solutions however, need to avoid the TBABr3 becoming soluble as the perbromide needs to remain insoluble for the battery to work as designed (create an insoluble perbromide to prevent self-discharge).
To make the solvent less polar by adding something else, we need to consider our potential choices and their polarity. We could add another solvent that doesn’t react with perbromide, like an alcohol, but we would need to be very careful with the amount to ensure that it does not make the perbromide soluble (since we know TBABr3 is slightly soluble in alcohols (see here)). We could also decrease the polarity by adding a polymer – like PEG 200 – which also has the benefit of decreasing the formation of dendrites in the Zinc anode. Both of these solutions are potential avenues for experimentation.
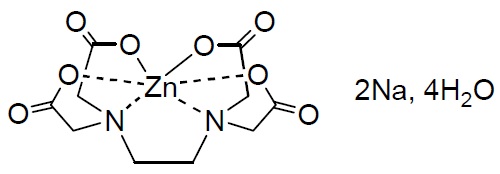
To decrease the polarity by masking the Zinc ion we can use a chelating agent that can react with the Zinc in order to reduce its effect on the dielectric constant of the medium. We could do this by replacing ZnBr2 with ZnEDTANa2 which replace bromides by the Zn(EDTA)-2 complex and requires the addition of two sodium ions, which are bound to be significantly less polar than the Zn+2 cation. However this would imply we would have less bromide available, so it might require the addition of NaBr to recover the equivalent moles of bromide we have lost. Alternatively we can also just add NaH2EDTA2 but we would require to make pH adjustments to the electrolyte, which is not something we would like to do. Additionally, the ZnEDTANa2 reagent is cheap and easily available – as it’s used as a fertilizer in agriculture – and the NaBr is also really low cost. This solution decreases the specific energy/power of the battery though, as the weight is increased by the use of additional reagents.
So there you have it, three potential experiments to try to make TBABr a viable sequestering agent for high energy/power density Zn-Br static batteries. Will they work? I plan to test them out one by one!