In my latest separator-free cells that use a PTFE o-ring spacer, I am now testing some additives to reduce dendrites and increase the life of the cells. A popular additive – PEG-200 – has proved not to be viable at a concentration of 20% due to large losses in the cell’s voltaic efficiency, moreover PEG-200 at a concentration of 1% offers little protection against dendrite formation. This last experiment tried a PEG-200 concentration of 6%, coupled with a small amount of NaCl to attempt to increase the conductivity and compensate for the loss caused by PEG-200.
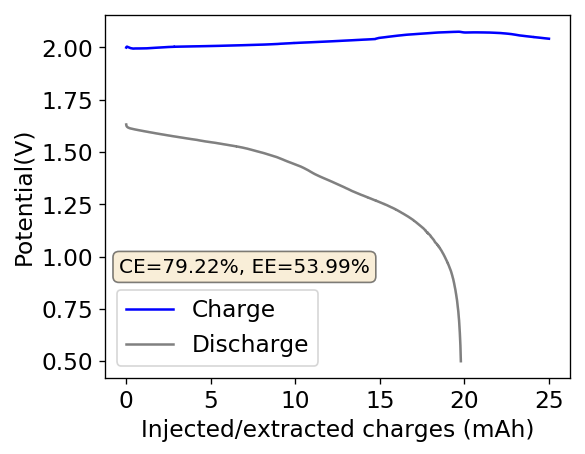
Above you can see the charge/discharge curve measured for this device. Compared to my previous devices the Coulombic and energy efficiencies have dropped significantly, with the most dramatic drop being in the energy efficiency. This value has dropped more than 10% relative with previous devices using a 1% PEG-200 concentration at the same zinc bromide concentration.
A device with this energy efficiency will not be viable, so I saw no need to cycle the battery multiple times. However to answer the question of whether zinc dendrites are formed or not, I then charged the cell a second time to 25mAh and opened the device, taking the picture of the anode shown below (I apologize if it seems out of focus, it wasn’t very easy for me to focus on such a small amount of space with my camera).

The picture above shows some interesting results. First, it was evident that there was absolutely no zinc dendrite formation, the plating was very crystalline and the electrode was flat with no protruding dendrites. Previous cells that had dendrite related failures show very tall dendrites that can easily be seen with the naked eye, even after only a few cycles. However you can also see several big and medium holes in the electrode where absolutely no zinc was deposited, this was caused by “air bubbles” trapped when the Swagelok cell is closed, which I haven’t been able to find a method to consistently remove. These bubbles remove so much of the surface area of the electrode that they can be responsible for significant losses in voltaic efficiency. Pre-wetting the electrode seems to be a viable method to ameliorate the issue but isn’t perfect.
In order to see if a cell like this can be viable, I am now testing a 6% PEG-200, 3M ZnBr2, 1.7M NaCl electrolyte, which should dramatically reduce the voltaic losses caused by the PEG-200 by increasing the conductivity of the electrolyte. Stay tuned for these results.
I don’t know if it would make a difference, but I have been using PEG 400 in my test batteries. It does seem to work in stopping dendrite formation, but until recently none of my electrodes survived more than a few charges before failing, so apart from visually looking through the side of the clear plastic and not seeing dendrites I can’t say if it is a good/bad thing for overall performance.
I’m using PEG 400 because a can get it from health shops on Ebay. Before using PEG 400, the cell would grow dendrites quickly and in gelled batteries the voltage would start to drop in very short dips indicating the dendrites were shorting out the cell. I haven’t been following any real recipe just a typical mishmash of items that seem to work (for now):
A gelled jar battery I made recently:
50ml ZnBr2 @ 3.5 MOL
50ml TBAB @ 0.25 MOL
50ml PEG 400
65ml NaBr @ 1 MOL
Mixed in Fumed Silica until a thick sticky paste formed.
Using Caplinq carbon filled HDPE I covered each side of a 13×1.5 cm copper mesh electrode with three layers. Two electrodes were made this way but I will be trying later with only one (the positive electrode) to see if that improves conductance by having one electrode as raw metal (but still survives so close to the bromine).
3D printed a disk with a 2x spiral pattern to hold the electrodes, make them curve around each other and fit into a jar with a 60mm opening. The distance between each electrode in the spiral was around 10mm.
The gel and the electrode assembly were placed in a jar and charged with a constant 150mA, which resulted in a 2.2V potential while charging. It would sit at 1.87V immediately after the charger was removed and after 12 hours was sitting around 1.786V (from self discharge).
Attaching a small motor that draws around 16mA the battery voltage would drop to 1.676V from the 1.786V it had from resting for 12 hours. The motor runs for many hours but I haven’t plotted a discharge curve as I’m currently focused on getting more current through the electrodes.
I know overall it’s not a very good test, but I didn’t have the Caplinq HDPE until recently and therefore all of my electrodes would not survive long enough to do useful tests.
I hopefully someone, somewhere, will find this info helpful.
Looking forward to your next post – cheers.
So gelling does not even help to stop the dendrites problems? If dendrites keep killing the battery then what is the permanent solution ? Also I see articles online about 2000cycles how do those who achieved 2000 cycles achieve this with dendrites problems???
As far as I know, gelling does not stop the dendrite problem, it makes the consequences of it worse in the sense that if the gel cannot self-heal, any dendrite damage might be irreversible. About those articles with 2000 cycles, you can make the cells last long if you have a cell without separator or gel that you discharge periodically by shorting the cell terminals – which will dissolve all dendrites – mechanically cleaning the dendrites every X cycles or putting enough additives in to eliminate dendrites, at the cost of dismal energy efficiency. But dendrites are a huge problem, a big reason why this technology does not have wide appeal.
Thanks for sharing the info about your setup! I wouldn’t expect there to be a huge difference between PEG-200 and PEG-400 in terms of how they reduce dendrites, but I would indeed expect PEG-400 to lower the conductivity of the solution even more.
From your recipe, your final concentrations are 0.8M ZnBr2, 0.3M NaBr, 0.05M TBAB, 23% PEG-400. Your final ZnBr2 concentration is actually on the lower end (0.8M), which is going to significantly decrease the energy density of your batteries. In my experience your ZnBr2 concentration should be at least 2M to enable you to reach energy densities above 20Wh/L. Your use of NaBr as a supporting electrolyte is interesting, I will try that and see how it affects my devices, maybe I can add enough to make a cell with 20% PEG-200 viable.
Thanks again for commenting and contributing 🙂
You’re welcome.
The gel batteries are a fun side project and I gel the electrolyte to contain it for handling while working with it. I had a ZnBr2 battery leak once and it was the worst bleach smell ever!
Recently I read that titanium in a water solution is resistant to halogens (except fluorine), so have ordered a small amount of titanium mesh and 0.1mm titanium foil to see if I can just place that straight into the electrolyte. If that works it should be more conductive the carbon filled HDPE.
Links to some of the titanium info:
https://www.timet.com/assets/local/documents/technicalmanuals/corrosion.pdf
https://www.researchgate.net/publication/289154283_What_you_need_to_know_about_MMO_coated_metal_anodes
Thanks for the comment Giancarlo! It is true that Ti is resistant to halogens, but under the oxidation potentials applied to the cathode it is definitely less resistant and will corrode with time if exposed to elemental Br2, you need to have quite efficient sequestering to ensure the Ti can survive for longer periods but even then, it is unlikely to make it past 100 cycles. Practically all commercial ZnBr2 flow batteries have used carbon materials for the cathode for this reason, including current collectors. However, definitely try it out and let us know if this is true or not, perhaps when using TBABr you have better luck.