This past week I did not post any new results for Zn-Br batteries. This is because I started to face significant reproducibility issues in my spacer based batteries with no separator. The image below shows you some of the typical curves I was getting from my batteries using PEG-200 containing solutions at a ZnBr2 concentration of 3M with different NaCl or NaBr additions. The battery started just fine – with CE values close to 90% – but fell sharply thereafter, with big increases in series resistance follower by large losses.
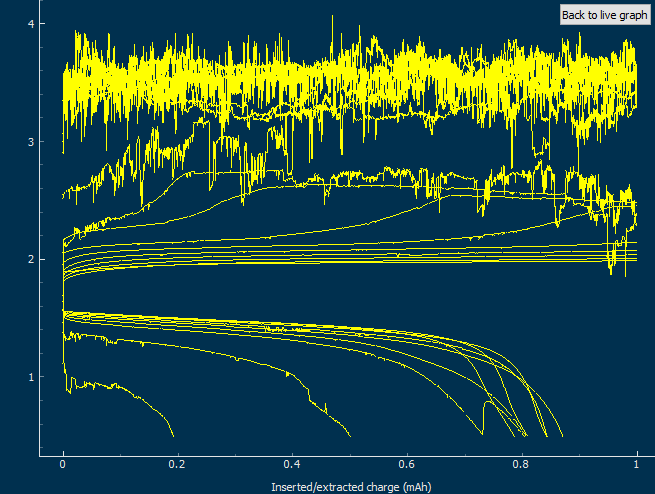
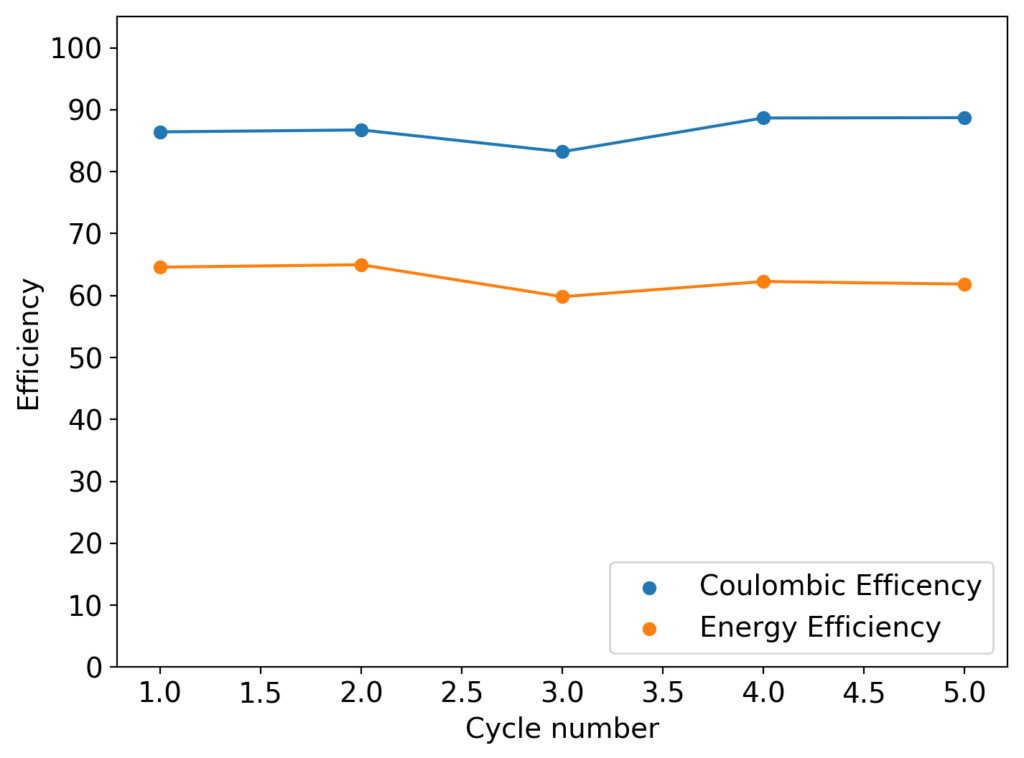
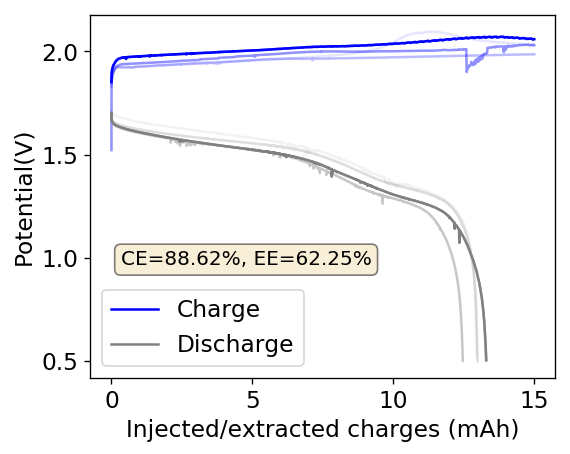
After a lot of investigation, the problem seems to be the adhesion of the Zn deposits to the anode’s graphite electrode. Even though the anode is always polished before every battery, the Zn deposits sometimes just “fall off” and – since there is no separator – that Zn falls to the cathode and is thereby lost and simply reacts slowly with the bromine. This was confirmed by moving again to a Zn metallic anode (0.2mm thickness) which didn’t show the above problems, as you can see in the curves below.
Although relatively normal CE and EE values were achieved for this battery configuration, dendrite formation was evident, both in the charge/discharge curves and after taking the battery apart (where dendrites were quite large). It is clear, both from NaCl and NaBr experiments, that additions of these supporting electrolytes contributes heavily to dendrite formation. It also seems pretty clear that going from 1% PEG-200 to 6% PEG-200 or higher doesn’t help enough with dendrite formation – they still form, even if a bit slower – but the heavy increase in series resistance is not worth the trade-off. If you try to add more PEG-200 and reduce the series resistance with NaBr or NaCl, then you just get the dendrites again.
From these experiments, it is now pretty clear why commercial ZnBr2 batteries do not use PEG-200 as an additive – at least in very large quantities – it might work to suppress formation of Zinc dendrites at lower ZnBr2 concentration (<1M) but at the concentrations required for energy density values greater than 30-40 Wh/L it just doesn’t seem to work well enough. Furthermore, while PEG-200 can be used with little effect in highly conductive KOH solutions that are used in some Zn chemistries (like Zn/Mn oxide batteries) it just doesn’t work when the electrolyte’s conductivity is significantly lower, such as is the case with ZnBr2 solutions.
All hope is not lost though. While PEG-200 by itself might not be able to prevent dendrites in this configuration, it is possible that low concentrations of PEG-200 plus other additives might have a synergistic enough effect to help us alleviate the problem. One such potential case is with the use of PEG-200 and Tween-20, which at 0.5% each, have shown to be both quite effective and synergistic at reducing Zinc dendrites. The experimentation continues!