As you saw on my previous post, I was able to generate pretty decent results with TMPhABr when using Zinc Bromide solutions at 0.5M with an addition of 0.25M of this quaternary ammonium salt. However it is pretty clear that at this concentration of Zinc Bromide the specific energy is too low, so I subsequently tried to reach higher efficiencies by trying higher concentrations of Zinc Bromide with a solid layer of TMPhABr (since at >0.5M of ZnBr2 the solubility drops too much). My experiments were done with the cell configuration showed below. The electrolyte also contained 1% of PEG-200 in order to prevent dendrite formation.
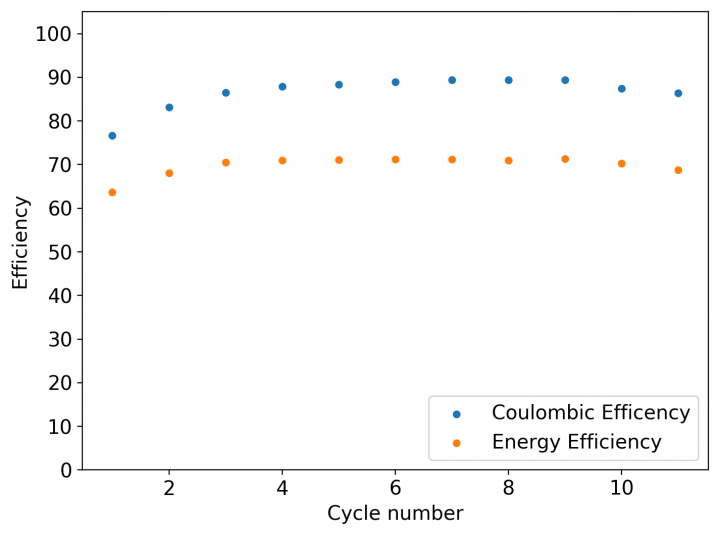
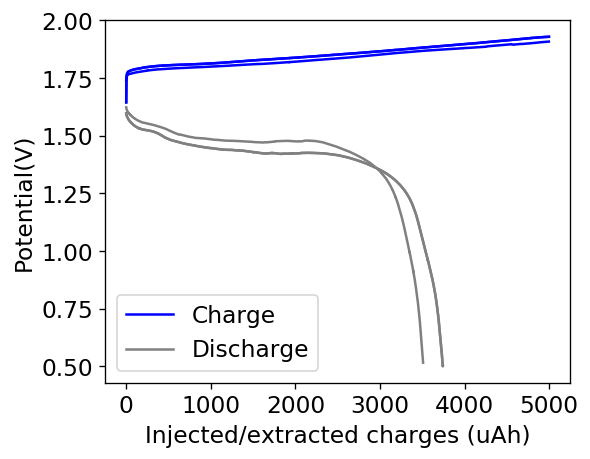
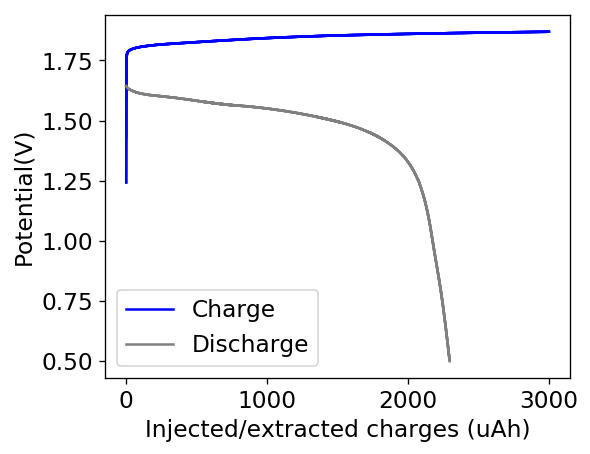
These experiments were quite successful, with a Coulombic efficiency of 86.41% and an energy efficiency of 68.74%. The capacity of these devices was increased by 4x over my previous experiments at 0.5M of ZnBr2 showing that the solid layer of TMPhABr does work in order to generate insoluble perbromides within the battery. However the battery performance did start to degrade at around the 10th cycle, so I stopped cycling the above battery to see if I could get better behavior at even higher Zinc Bromide concentrations since the increase in ZnBr2 concentration did show a reduction in the internal resistance of the battery.
The attempt to use higher concentrations at higher current densities were not very successful. Although the capacity was increased to around 10x of my initial battery, the problem was that both the Coulombic and energy efficiencies dropped to unacceptable levels. The charging voltage also saw substantial climbs – reaching almost 2V – which probably created a lot of unwanted reactions. The worst problem was however the zinc dendrite formation, which became apparent after I tried cycles at lower capacity and current density for the same cell. You can see below that at the fourth cycle the charge voltage drops suddenly and then the discharge is extremely inefficient. This is because dendrites have pierced the separator effectively shorting the battery.
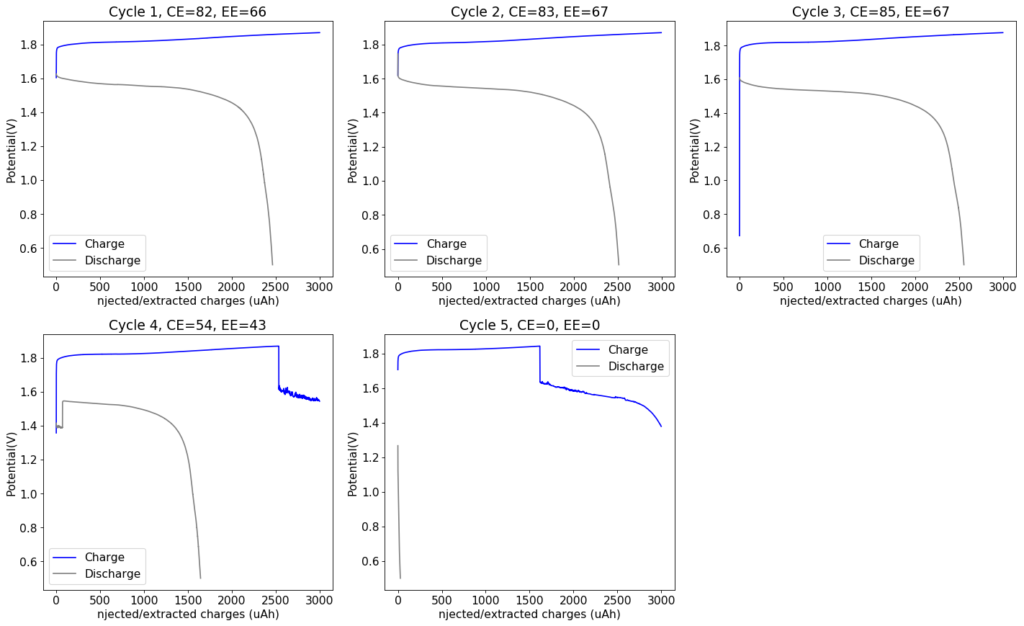
This dendrite issue is one of the most important problems in Zinc-Bromine batteries – both flow and static – and one of the reasons why rechargeable Zinc chemistries have not been massively adopted thus far. If the above batteries are to be practical, I need to find a setup that provides both high capacity – which means a 3M ZnBr2 electrolyte – with the elimination of Zinc dendrites. The addition of PEG-200 helps, but it is clearly not enough to eliminate this issue. Upon opening the above battery, it was evident that dendrites had completely pierced through the entire separator and shorted the electrodes.
One hypothesis I have is that local formation of Zinc dendrites should be hindered by high local TMPhABr concentrations (since they do not form when high amounts of this are dissolved) so a potential solution is to create another solid layer of the TMPhABr next to the Zinc anode (as shown below). I am currently testing the battery configuration shown below to evaluate this hypothesis.
Another issue that has been pointed out to be is the absence of additional support electrolyte, so I am planning to test ammonium sulfate at 2M to see how this modifies the behavior of my batteries at these higher capacities. Ammonium ions will turn my battery more acidic, so I am expecting some losses in Coulombic efficiency at higher current densities from a more favorable hydrogen evolution potential.
Great to see someone else looking into this type of battery. I too have recently started playing around with zinc bromide batteries after finding Robert’s youtube channel. I am also using TBAB to capture the bromine. Not being a chemist I have been focusing more on practical construction of the battery and electrodes, trying to squeeze as much current through the electrodes by finding the lowest possible electrical resistance, but most practical way to make the them.
Keep up on it, I’m looking forward to seeing your future posts.
Cheers.
Thanks for posting Giancarlo! Do you have any charge/discharge curves of your experiments you could share with us? I tried a lot to make TBAB work, but could never get those batteries to behave properly due to the very low solubility of TBAB in the presence of ZnBr2. Also have you had issues with zinc dendrites? Let us know!
I’m still shopping for a device to do the measurements for the charge/discharge curves. Currently looking at the ZKETECH devices like the EBD-A20H
https://www.seeedstudio.com/ZKETECH-EBD-A20H-AC-Electronic-Load-Battery-Capacity-Tester-Powe-Supply-Tester-30V-20A-200W-p-4521.html
I have found the electrodes to be biggest issue because I’ll be using the batteries for night time power for my future home solar system. The power draw from the batteries to supply the inverter will be greater than 150 amps @ 48 volts so having the lowest impedance is critical.
I have had the TBAB issues of solubility but the electrodes I’m working on are a combination of weaved copper strip dipped a few times in a dissolved plastic (ABS/Polycaprolactone/Polysyrene etc) with graphite and slices/layers of carbon felt/cloth soaked in TBAB. Any TBAB that has come out in solution is still there to be complexed once charging is underway and tends to collect in the carbon cloth at the bottom of the battery.
Because my batteries are for a house and won’t be moving around, having a very liquid battery isn’t a problem, and I tend to use oil on top to stop any bromine from escaping (this works well but some oils are better then others).
The dendrites are a problem but I haven’t worried too much about them yet as I’m only been doing this for a couple of months and only just got the chemicals like TBAB and PEG to play with. In time I’m hoping to find a workable solution. The PEG I managed to get is PEG 400 so it will be interesting to see how that behaves Vs the PEG 200 everyone else is using.
I’ll definitely be letting you know how to all goes in time and I’ll check in on your work to see if you have had any luck at your end.
Cheers.
Awesome recommendation on the EBD-A20H, I had been looking for something to run tests when I move to higher capacities, this seems to be able to do the job for a very low price. I am currently using a DIY solution to test my batteries (https://www.sciencedirect.com/science/article/pii/S2468067217300317), which is great for the small batteries I am testing, all the software it uses is also open-source, so I can easily modify it to carry out any experiments I want.
About your electrodes, you might want to try this graphite carbon felt (https://www.ceramaterials.com/product-category/carbon-graphite-products/specialty-felt/) it has very high conductivity and surface area and its price is quite moderate, especially if you get 2K USD of it.
Thanks again for your comments! Let us know how about your progress as well!