In July 2021, I wrote about my goal to reproduce the results of a paper using a high surface area carbon, loaded with elemental iodine, to create a practical Zinc-Iodine battery.
This was not successful because my material of choice – a GFE-1 cathode – did not absorb any iodine when placed in an iodine chamber at 55C for 10 minutes. This is because the cathode material is likely already passivated, as it has been in air for more than a year now, so activation would likely be necessary to get it to load with Iodine. Performing this process is outside my current technical possibilities (no ovens or vacuum chambers available).
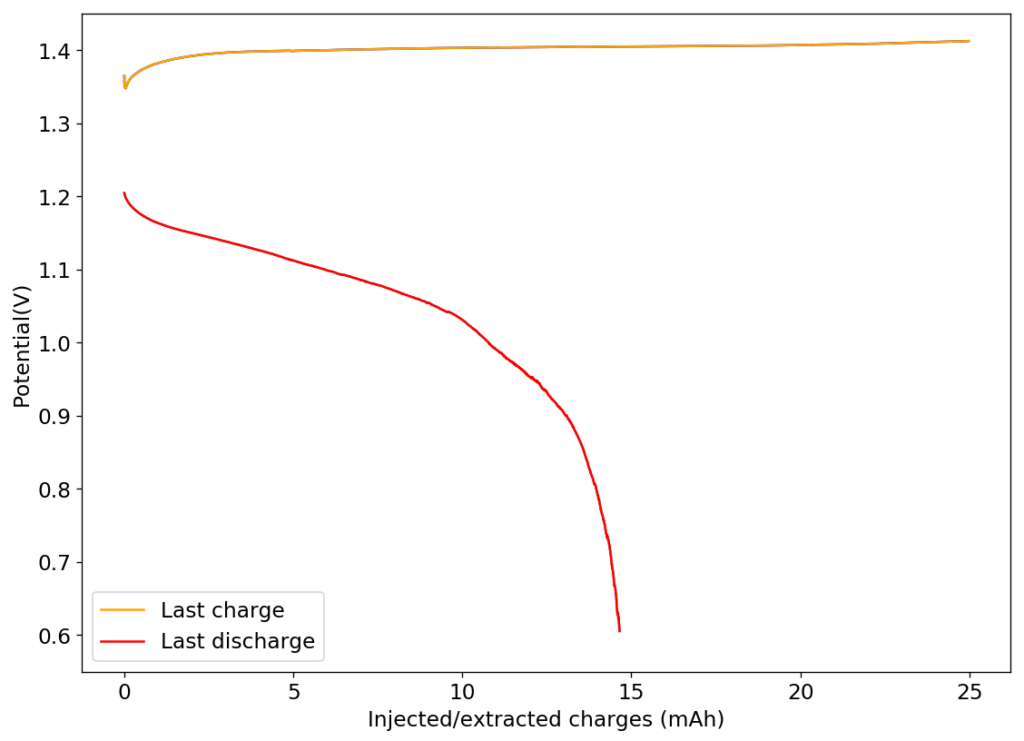
Instead of continuing down this path, I decided to create a battery using Zinc Iodide. This chemical is hard to get though, so I synthesized it from metallic Zinc and elemental Iodine. With the 2.5M solution ZnI2 I created – concentration determined by its density – I then proceeded to create batteries in my Swagelok cell configuration. The best result I got, where energy density was around 29 Wh/L, are shown above.
Despite the above, I was never able to achieve high CE or EE values, with the values for my batteries at >20Wh/L capacities being around 55% for CE and 35% for EE. Low coulombic efficiency happened because of diffusion of elemental iodine away from the cathode – through the formation and diffusion of I3– – as evidenced by the strong coloring of the fiber glass separators when taking the batteries apart. In a similar fashion to Zn-Br batteries, the elemental halogen diffuses and this inevitably lowers the CE, EE and increases the self-discharge of the battery. Even the high surface GFE-1 carbon felt, is just uncapable of holding to the halogen on its own.
Besides making most properties of the batteries worse, the diffusion eventually kills the battery, as so much reagent is lost per cycle that at some point the battery is simply unable to properly function, as any generated zinc is consumed by Iodine that reaches the anode.
I have tried several things so far to eliminate the problem. Thicker carbon material, more dilute ZnI2, TBABr as a complexing agent, none of these have worked, since I3– forms very efficiently under any excess of I– in the solution, which is inevitable as when the reaction starts there is ample iodide in solution. Adding TBAB does precipitate the TBAI3 salt, but this greatly increases the series resistance of the battery.
Is there anyway to stop the I2 from becoming I3– and migrating? Can we somehow plate elemental I2 at the cathode and avoid self-discharge and losses in CE and EE? Stay tuned for more research results.
“… my material of choice – a GFE-1 cathode – did not absorb any iodine when placed in an iodine chamber at 55C for 10 minutes. This is because the cathode material is likely already passivated, as it has been in air for more than a year now, so activation would likely be necessary to get it to load with Iodine. Performing this process is outside my current technical possibilities (no ovens or vacuum chambers available)…”
What I’m going to say might be dumb but…you want a “high surface area carbon”. I’ve thought about this and I’m also interested in Terra-preta which is soil amended with pyrolyzed carbon which can be made very easily. You put wood in a barrel with holes punched in the bottom. This is then put in another barrel and a fire is started in it such that the inner barrel with wood in it is cooked and the volatiles are cooked off further adding to the flames. Wood gas. I’m sure you know what that is. Anyways I searched and could not find any info on what a “GFE-1 cathode”is, (what is it I see no reference except your site), hence my possible stupidity in this.
“If” it’s just a piece of pyrolyzed carbon then you can make this stuff easily. Maybe you could put your cathode in a metal can, like a paint can, with a hole punched in the bottom. Maybe add a couple pieces of charcoal to make sure the air is driven out and the can filled with CO2 and it would drive off all the gases in it . Build a fire under it until all the gas is driven off. Looking at “DIY Biochar” videos for instructions.
“If” a “GFE-1 cathode” is really just a open celled carbon structure then I expect you could make one of these out of cheap pine boards treated like biochar as above.
I did see noted here,
https://en.wikipedia.org/wiki/Activated_carbon
that,”…Activated carbon adsorbs iodine very well. The iodine capacity, mg/g, (ASTM D28 Standard Method test) may be used as an indication of total surface area. …”
I confused about whether the resistivity would not be too high. I know Graphitic carbon conducts well and so does carbon black but I don’t know about bio-char. I see on this page on carbon resistivity
https://hypertextbook.com/facts/2004/AfricaBelgrave.shtml
it’s 35 µΩm which doesn’t sound like much to me. How would you connect the carbon to a copper terminal? Could you embed copper wires in it and not have them electrolyzed away???
The point here is that if you want something cheap, and don’t we all, then thin sheets of wood pyrolyzed in metal cans is about as cheap as you can get and you have a very high surface area to work with if you can only get the stuff to conduct.
My apologies if this is nonsensical.
The GFE-1 cathode is a very high surface area conductive carbon felt (https://www.ceramaterials.com/product/gfe-1/) probably one of the best carbon materials you can buy. Surface area is >1000m2/g. The problem is not that the material has a low surface area, but that the high surface area is passivated by things the carbon absorbs from the air. This felt – or any other carbon material for that matter – needs to be “reactivated” if you want it to uptake something like iodine readily. Especially if the carbon material was made a long time ago.
Note that I don’t have ovens, wooden stoves, places to burn wood, etc. So I have to work with commercially available carbon materials that are standardized and well sourced. Materials produced in artisanal ways might fit the bill, but they might be hard to reproduce in a reliable manner and demand a lot of space and craft from people making them. This is great for people who want to explore these possibilities, but definitely not interesting for me.
I forgot to add, thank you for doing this and posting your results of your experiments. It’s great.
Don’t know if you ever tried it but apparently you can us ZnSO4 + 1.3KI + 0.67KBr to prevent the I3- forming getting I2Br- trihalide instead which is more readily soluble. Just an FYI from my research.
you work is amazing.. loving it.. and learning from it.