During my journey to understand and built better Zn-Br batteries I have constructed batteries using both separator and separator-less setups. In the separator containing setups there are layers of non-woven fiberglass tissue paper between the anode and cathode while the separator-less setups use PTFE o-rings as spacers to maintain the distance between the cathode and anode constant. Through my experience with both of these setups I have gained some useful experience that I am going to share within this post. More specifically I will be showing you a list of pros/cons for each architecture.
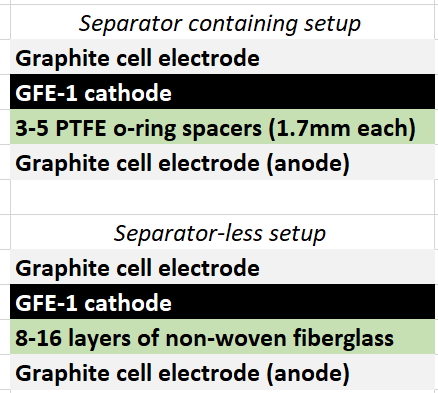
Non-woven fiberglass separator containing setup
Pros:
- Electrolyte is absorbed within the separator, therefore the cell can be moved freely for short periods of time without issues.
- Since the separator is solid it can be compressed more, leading to better battery characteristics.
- Compression is homogenous through the entire cell, which makes devices more reproducible.
- There is no spacer to keep electrode distance so all the effective cross-section can be used which leads to a more efficient use of cell volume.
- Slower diffusion of bromine across the device, which leads to slower self-discharge.
- Easily scalable.
Cons:
- Cell cannot be easily maintained or electrolyte easily replaced.
- Dendrites irreversibly damage the separator since the separator is physically torn by the zinc dendrites.
- There can be significant edge effects at the edge of the separator, as charges flow more efficiently around these areas since their interaction with the separator is weaker.
- Absence of important edge effects in the device as diffusion speed is likely constant through the cross-section.
PTFE o-ring spacer setup
Pros:
- Faster ion diffusion due to the absence of any additional material between most anode and cathode which can lead to better charge characteristics.
- Electrolyte can be replaced easily by opening up the cell and refilling or washing the cell components.
- Zinc dendrites are not irreversibly destructive, causing only self-discharge issues as they reach into the cathode. When the battery is at rest dendrites tend to re-dissolve as bromine diffuses from the cathode to the anode.
Cons:
- Compression of a spacer setup can cause a carbon felt cathode to be compressed into the o-ring, effectively getting closer and closer to the anode and sometimes cutting the cathode material. This effect is difficult to control well, which causes reproducibility problems between devices.
- The devices cannot be moved because sloshing of the solution can cause important issues, such as self-discharge, to accelerate exponentially.
- Due to the spacer taking some volume in the device, the amount of usable coss-section decreases, which causes substantial losses in device energy density.
- Not easily scalable as electrodes will tend to sag when larger cross-sections are used, therefore requiring the design of a scalable spacer design (like a PTFE grid) which is likely to be expensive.
After thinking a lot about the above characteristics, it seems to me that the main important disadvantage of the spacer setup is its vulnerability to become damaged irreversibly by the appearance of zinc dendrites. If these are not a substantial issues, then a fiberglass separator setup would be significantly better approach – both in terms of cost, reproducibility, scalability and performance – relative to a PTFE spacer based setup using no separators.
Let me know what you guys thing about the above!
I have only built three types of ZnBr2 batteries:
1. Static liquid, no separator, Anode on top.
2. Static liquid, oasis foam separator, Anode on top.
3. Gelled type.
All were tried with various types of electrodes.
Never tried using a fibre glass separator, but in the liquid type of battery I would like to try it to see if it slows down the self discharge rate without making the internal resistance worse (like the oasis foam did). The dendrites grew down the side of the oasis foam when I just had it rammed in (probably finding the easiest/most conductive way past the foam). Later I hot-glued the edges of the foam to the container to seal it better, but that trapped in the gasses being made and the foam crack and split anyway.
My gelled batteries tend to last longer than my liquid ones but I haven’t been pushing any of my recent batteries too hard as I’m waiting for my tester to arrive so I can to more quantitative testing.
So in reference to your question above; ever thought of trying a gel cell? I may have been using too much PEG-400 and too low a dose of ZnBr2 but none of my gel batteries died from dendrites. Maybe the gel interferes with the formation of dendrites in some way?
Thanks for your comments Giancarlo! I haven’t tried gel batteries, not because I have anything against them but because I have been busy trying simpler types of batteries (non-woven fiberglass separators and PTFE o-ring spacers). Once I have an example where the chemistry is stable I might try to create a gel-type battery. Especially if your more quantitative results are interesting.
I wouldn’t expect gels to reduce dendrite formation though, at least from the research I have read on gelled batteries this doesn’t seem apparent. I think that the lack of dendrites you have observed is most likely related with your lower ZnBr2 concentration and your use of PEG-400, both which contribute to a significantly lower amount of dendrites. Looking forward to hearing about your quantitative findings!
Hi, I’m an electrical engineer, and interested (hoby only) in constructing some ZnBr2 batteries, thought I’d inquire as to your success and fails, doesn’t make sense to repeat others’ mistakes. Would you mind sharing the trials you;ve made, maybe some data that resulted? Or point me in a direction such info (yours or others’) is written? Thanks so much.
I’ll be happy to reply back with my own results once I’ve made some progress as well.
Bill K
Hi Bill,
Thanks for commenting. My trials are mostly detailed within my blog, if you read through my blog posts you’ll have a good idea of what I have done and the results I have obtained. I would suggest that you get some equipment to properly characterize your devices as people are often unable to progress due to a lack of a proper quantitative approach to these batteries (for example, lack of ability to measure charge/discharge curves). Let me know if you have any specific questions,
Best Regards,
Daniel
Hi Daniel,
Great blog you have here, I’m going through a similar process myself at the moment in testing out this battery chemistry.
I have read a study recently about using tetrapropylammonium bromide as an additive in a zinc bromide battery using fibreglass seperators and they had promising results, it completely inhibited the growth of zinc dendrites, eliminated self discharge and improved the performance of the cell.
Short of the ionic selective membrane tech that is under lock and key at Gelion/University of Sydney i think this is the most promising stuff I have come across in overcoming the limitations of the zinc bromide electrolyte.
I am a novice chemist at best and don’t know what would be involved in the synthesis of tetrapropylammonium bromide (as much as I have searched) but needless to say the chemical companies that stock it won’t sell it to a private individual despite it not being any sort of precursor to anything illegal.
Hi Mike,
Thanks for commenting! The Chinese paper you’re probably referring to, is sadly pure smoke, I encourage you to read my blog post on why this paper is nothing to get excited about (https://chemisting.com/2020/09/12/zinc-bromine-batteries-can-they-really-be-that-good/). In summary, the batteries only appear good because they normalize all performance to just 3mg of cathode mass, which is ridiculous. If you normalize to the actual mass they should use, the performance values are 100x smaller. The TPABr is not a great complexing agent for these batteries either, it is highly insoluble in concentrated ZnBr2 solutions, which limits the maximum concentration of ZnBr2 to around 0.5M, which is just not practical for high density batteries (we need at least >2M for batteries to come even close to lead acid capacity values).
Also, the gel battery technology from Sydney has been patented, so it is all public information (https://patents.google.com/patent/EP3103156A1/en). These batteries are however much more complicated than your simple static ZnBr2 batteries, they require a variety of custom ionic liquids that are expensive to buy or make, probably the reason why this technology hasn’t been commercialized yet.
All-in-all, the problem with Zn-Br batteries remains very much alive and the problems inherent to the technology remain largely unsolved. A cheap solution to deal with dendrite formation, self-discharge and sequestration in a static setup is still nowhere to be found. Even very simple setups like the Princeton minimal architecture battery have irreversible damage problems dealing with hydrogen evolution.
I encourage you to build and quantify some of these batteries, so that you can get an idea of the magnitude of these difficulties. My swagelok cell setup is a simple way for anyone to get started with this battery technology.
Thanks again for writing!
Couldn’t agree more.