In order to study the chemistry of Zinc-Bromine batteries I have been using a swagelok cell that I bought from China that has a central Teflon body with stainless steel electrodes. This has been problematic due to the reactivity of these electrodes with the elemental bromine and tribromide salts produced in the battery, requiring the use of some “improvisation” in order to make the batteries work.
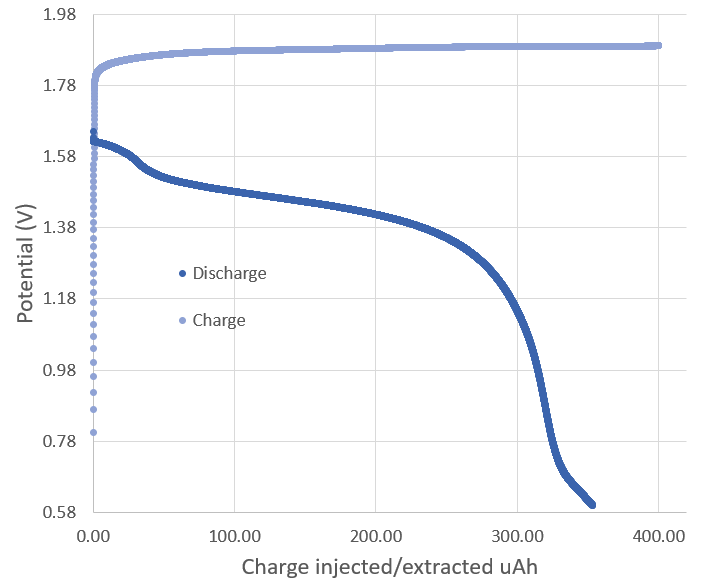
To be able to generate the necessary chemical reactions without interference from the stainless steel I have coated the electrodes with some conductive HDPE I have, which has a relatively high volume resistance of around 10K ohm. The charge/discharge curve above shows you the type of measurements I have been able to achieve with this setup, with the above curve having a Coulombic efficiency of 88%.
This is lower than what I could achieve with the copper anode I was using previously (which gave me around 96%), but note how the charging voltage is lower and the discharge voltage higher, meaning that the overall energy efficiency is significantly better (around double). This is however not because of the zinc anode, but because with the conductive HDPE now covering both electrodes, I have now been able to tighten the cell more and achieve a lower overall internal resistance.
However the still relative low energy efficiency and voltage drop when going from charge to discharge are still pointing to significant sources of efficiency loss, possibly from the 10K ohm resistance that the conductive HDPE is giving. The weird shapes at the beginning and end of the discharge curve are also pointing to more than one chemical reaction happening, probably because the electrodes are still somehow interfering with the chemistry (maybe some micro holes in the conductive HDPE at points of stress are exposing the electrodes underneath). For this reason I have decided to get graphite electrodes for the Swagelok cell, which I will build from these 0.5 inch diameter graphite rods I found.
I am also going to change the current carbon felt cathode for carbon paper electrodes – which are on the way – but I will refrain from using the paper until I can perform some tests using actual graphite electrodes that are guaranteed to be free of any pesky side reactions, with way less resistance than this conductive HDPE.