Secondary Zn-Br batteries suffer from a huge problem of self-discharge due to the formation of elemental Bromine which, although largely insoluble in water, is soluble enough to migrate through the cell and react with the zinc anode, effectively self-discharging the cell.
To circumvent this issue, researchers have used chemicals that sequester the produced bromine into a product that has even less affinity for water — an insoluble or immiscible perbromide. In flow batteries this is done to generate a liquid phase that is immiscible with water, since it still needs to be a liquid to allow proper flow of the reagent. In static batteries this is undesirable, because a liquid is still able to flow through the cell and react with the Zn anode.
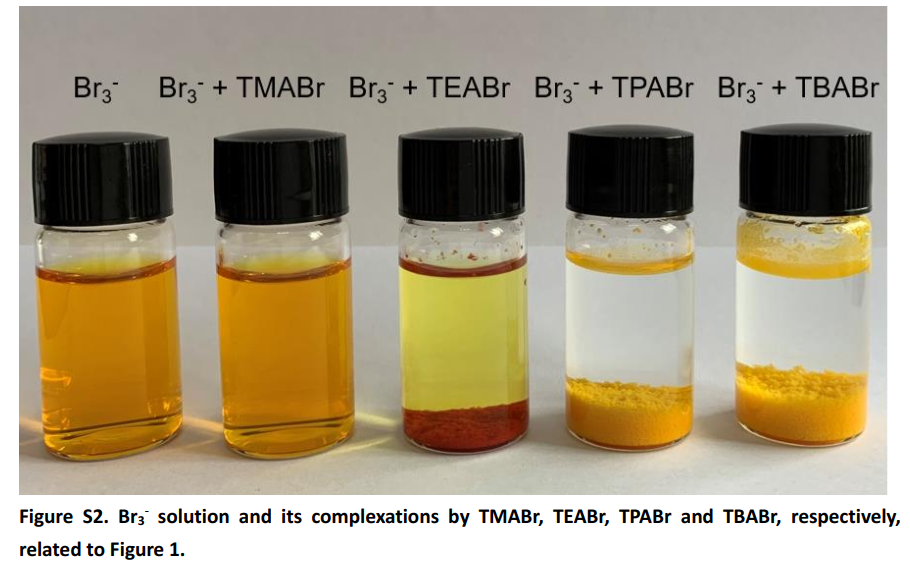
The 2020 Chinese paper we’ve discussed previously in this blog goes around this problem by using a sequestering agent that forms an insoluble perbromide, tetrapropylammonium bromide (TPABr). Notably the paper uses TPABr instead of tetrabutylammonium bromide (TBABr) which is almost an order of magnitude cheaper due to its significantly wider array of industrial uses compared to TPABr. Not only that, but the TBABr perbromide is even more insoluble, so the chemistry should be even better, right?
It is worth noting that they are aware of the above facts. You can see this in the image above – taken from the supporting information of the paper – where they clearly show TBABr forms an insoluble perbromide. So why did they choose to go with a significantly more expensive chemical (TPABr) and not use TBABr when its the obvious choice from a practical standpoint?

The problem – which I have lived through experimentally – is that the solubility of TBABr in the presence of ZnBr2 is quite terrible. The TBABr is extremely soluble in water – you can easily prepare a 50% solution by weight in distilled water – but it precipitates back very aggressively when put it into contact with a solution of zinc bromide. The image above shows you what happens when you mix a 1M solution of TBABr with a 0.5M solution of ZnBr2. The authors of the paper probably saw this issue and immediately recognized this as a potential problem for their batteries, my intuition is that they did run and have results for some cells using TBABr, but the results were probably so much worse than those of TPABr, due to this solubility issue, that they simply did not publish them.
The TPABr is most probably a significantly better sequestering agent because it’s likely significantly more soluble than TPABr in Zinc Bromide solutions. This agent is however unlikely to be soluble enough to support very large capacity solutions (>= 2M ZnBr2).
As I mentioned on a previous post, a better sequestering agent must allow for large solubility, be commercially available and form an insoluble perbromide. The only candidate I can think of to fulfill this role would be trimethylphenylammonium bromide (TMPhABr). I might be tempted enough to test it to order some from Alibaba if I can get a low quantity order for a reasonable price!