I have mentioned how the usability of TBABr in Zn-Br batteries is limited due to the poor solubility of TBABr in the presence of large concentrations of zinc bromide. In my experiments the most concentrated solution I was able to get was around 0.1M ZnBr2 + 0.1M TBABr. This is problematic since we aren’t going to achieve high specific energy or power values with an electrolyte that is this dilute in terms of Zn concentration. However, what if we put a suspension into the cell as the electrolyte?

When the cell is charging, the concentration of TBABr in the electrolyte will go down as TBABr3 precipitates out of solution. However, if there is extra TBABr within the cell, that solid will dissolve to replace the TBABr that precipitated. When the cell is discharged, the process will reverse, TBABr3 will redissolve and some TBABr will precipitate again as it is pushed out of solution by the perbromide that needs to go back into solution. The conductvity of the solution should be less affected, because it will only be reduced as a function of the loss of ZnBr2, without an actual loss of TBABr. The problem of course, is that there will be some solid TBABr in the cell, which is likely to increase the series resistance of the cell (because the solid salt is not a conductor).
How do we achieve this? To do this I first put 0.720g of ZnBr2 into a 10mL volumetric flask, then dissolved that into 1mL of distilled water. I then added as much 1M TBABr solution as needed to fill the volumetric flask to 10mL. The total concentration of ZnBr2 is around 0.33M but a lot of “solid” precipitates out of solution, forming a high viscosity phase with the consistency of honey that is made almost entirely out of TBABr. If we agitate the flask, this phase gets suspended into solution quite easily, forming a cloudy suspension (see image above).
I then built a battery within my graphite electrode Swagelok cell using a zinc anode, 8 layers of fiber glass separator and a carbon felt cathode. I then added 100uL of the above prepared suspension right after agitating the flask vigorously, allowing the material to wick through the cell for a minute before closing the Swagelok cell.
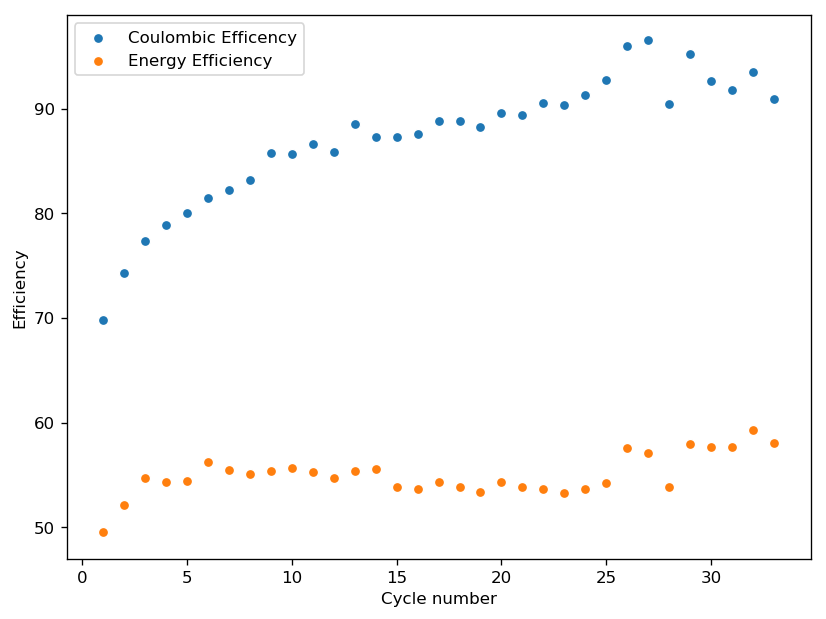
I have since started doing charge/discharge cycles of this cell with very interesting results (see above). The cell initially had relatively low coulombic efficiency (CE) and energy efficiency (EE) values, but these started improving as the cell was cycled. My hypothesis is that – per my previous explanation – the solid is first randomly distributed within the cell but gets organized and deposited within the cathode as the number of charge/discharge cycles increases. I believe this greatly improves the formation of the TBABr3 within the cathode and prevents the solubilization of the perbromide, which reduces self-discharge and therefore increases the cell’s efficiency.
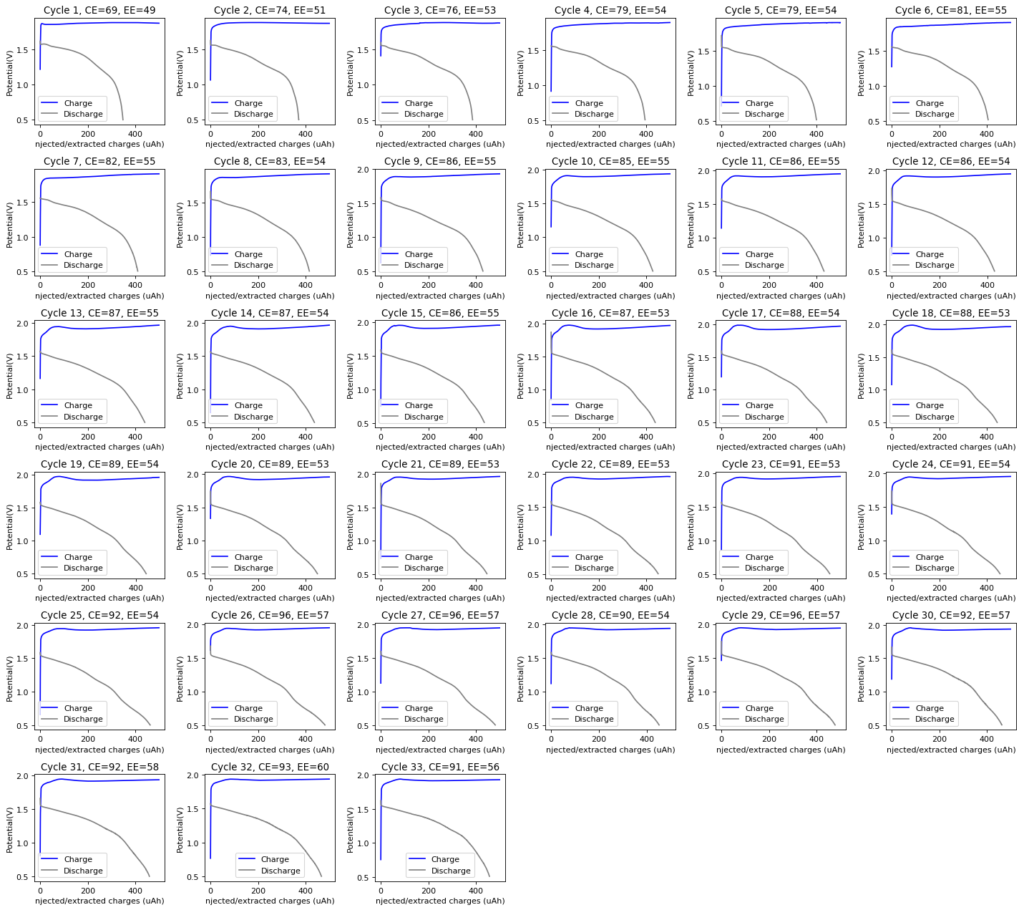
I believe we can see some experimental evidence for this hypothesis as we see a “shoulder” emerge at the start of the charge phase as the number of cycles increases. I think this is consistent with a significant amount of TBABr deposited close to the cathode interface after discharge, which creates a higher resistance to current flow that subsides as the TBABr3 starts forming and this TBABr dissolves back into solution. This is of course an interpretation based on very limited information and I would be thrilled to know what any of you think about the evolution of the charge/discharge curves and what you believe they are telling us. I will continue cycling this cell during the next 2-3 days, to see how the cell stabilizes and whether the CE and EE start going down after.
With that said, it seems pretty clear that TBABr by itself is not going to be an adequate sequestering agent. I will be trying to use PEG200 to increase its solubility – as discussed in some of my previous posts – but I also already ordered TMPhABr (trimethylphenylammonium bromide) as I believe this will be a way better sequestering agent for these devices.