As my avid reader Giancarlo pointed out in the comment section a few posts back, many of the big problems of the Zn-Br battery system seem to be caused by the use of an aqueous electrolyte. Hydrogen evolution and voltaic losses due to the use of insoluble or immiscible bromine sequestering agents are some of the biggest issues that are inevitably related with water. Changing to a non-aqueous solvent can help solve some problems, although some others are created.
An organic solvent to replace water in Zn-Br batteries would need to be aprotic and to allow for the creation of substantially conductive solutions using ZnBr2. The issue with these organic solvents is that they are also extremely friendly to bromine, most of them being infinitely miscible with elemental bromine. This means that a battery built using these highly polar, aprotic solvents, would discharge significantly faster, as bromine would be significantly more likely to migrate to the anode. Sequestering agents would not be usable as these agents and their tribromides are incredibly soluble in these solvents as well.

However, this property can be exploited to solve part of the problems of the Zn-Br battery, at least the part dealing with voltaic losses related with the sequestering agents in water. This paper from 1988 shows how propionitrile can be used within a battery to sequester bromine and prevent its migration through the cell. In this device, an aqueous anolyte is used with an organic catholyte to trap bromine near the cathode. Since bromine has such a high affinity for propionitrile it will tend to stay in the organic section of the device, preventing movements towards the aqueous layer and providing more efficient confining and higher conductivity compared with some common sequestering agents. This is the only paper I could find that discusses the testing of Zn-Br batteries with a non-aqueous solvent that is not an ionic liquid. There are however, no papers I could find where the anolyte is replaced by an ionic liquid as well.
Propionitrile – and nitriles in general – are nasty solvents though. They are significantly toxic and we wouldn’t want to use them for DIY home batteries due to these issues, especially if we’re going to be experimenting and having close contact with their solutions. For this reason I will avoid their use and will instead use the non-toxic aprotic polar solvent, propylene carbonate. This solvent can also be bought pretty easily on the internet (I got mine here).
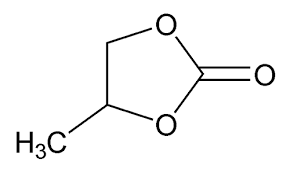
Propylene carbonate can form highly conductive solutions with some salts, it is reasonable to predict that both the quaternary ammonium salt I have (TMPhABr and TBABr) will be soluble in it, as well as ZnBr2. I have no idea about whether Br2 or perbromides are soluble in it though, but it is reasonable to expect Br2 to be highly soluble in it because of how other, similar solvents, behave. There is some precedent for a battery fully constructed with a metal bromide using entirely propylene carbonate, see this paper, so it might actually be possible to use propylene carbonate by itself, although this is probably not possible without a suitable membrane separator.
We know the diffusion coefficient of Br2 in propylene carbonate to be 3.41×10−6 cm2 s−1 (1) while the coefficient in water is 1.18×10−5 cm2 s−1 (2), this is in part because of the higher viscosity of the organic solvent. This means that diffusion of Br2 in propylene carbonate will be more than 5x slower, which might be enough to create a functional battery with limited self-discharge, especially if this coefficient can be reduced further with the addition of the quaternary ammonium salts.
Even if the creation of a battery using entirely propylene carbonate is not possible, it might be the case that a highly concentrated TMPhABr or TBABr solution in this solvent will be immiscible with a 3M solution of ZnBr2 in water. If this is the case then a split approach with a bottom organic solvent and a top aqueous solvent might be possible, although this will depend largely on what the final density values actually are.
In any case, I have now ordered some propylene carbonate and will be carrying out some tests with it during the next couple of weeks.
While it adds to the resistance of the battery maybe gelling the propylene carbonate with fumed silica to limit the diffusion. If the anode and cathode are close together lowering the resistance and limiting the dentrites with the Tween 20 & PEG 200 mix to prevent short circuiting, you could have a winning combination.
I have had good results from gelling my batteries. While a liquid battery would be best for a battery that never moves around, a gelled battery would be great for portable applications.
The site you mentioned for the propylene carbonate has sold out 🙁 already (this stuff is popular)… I’ll have to keep looking.
Thanks again for the work you are doing.
Cheers.
Hello what process do you use to gel your zinc bromine battery?
I just add fumed silica to the electrolyte until it is thick enough not to move when holding it’s container sideways. It should stick to anything you are mixing it with and not fall off. Even this thick, I can still use it in a plastic syringe and inject it into a battery.
You should be able to get “fumed silica” from fiber glass suppliers as it is used as a resin filler. Note, don’t order “silica fume”, that is something completely different.
Thanks for your comment! The idea of gelling the electrolyte is pretty interesting, how big of an increase in charging voltage have you had by doing this? Has this made any difference in the case of dendrites or have they formed the same anyway? I am afraid the gelling might seriously affect energy efficiency, although it’s definitely not an expensive thing to try. This might actually help with issues related with evaporation/leakage that tend to be common when I use fiberglass separators. Thanks in advance for your answers!
My latest gelled battery started charging with 2.35V@200mA. Later on (half hour or so) the voltage was around 2.15V@200mA, and only went up slowly after that but never reached 2.35V again (on the first charge) before the charging was complete. Charging was stopped periodically to check the static off charge voltage. Once an off charge of 1.85V was reached a DC motor that draws around 12.5mA was connected. This resulted in the voltage dropping under load to 1.80V immediately and sat in the 1.7V range for over an hour. The battery is now 3 days old and is still going good.
I should say that you shouldn’t let the battery drain down below 1.1V. I found it will need 2.5V@200mA at the start of charging, but if you cycle the cell by running the motor for a minute after charging for 10 minutes the battery slowly starts to behave like before.
I really have to get a proper testing setup. I just use the same DC motor and constant current charging to gauge how each battery is performing and charge it again and again each day until it fails in some way.
I have made a few different gel batteries with a spiral type being the best for getting as much electrode as possible into the cell. This particular battery is made of two copper pipes (1 inch and 0.5 inch) 5cm long, nested inside of each other resulting in a 4mm difference between the outer and inner pipes. The inner pipe has the carbon filled HDPE ironed onto it to protect it from the bromine that will form around it. The outer pipe is left as is. Two caps made out of 6mm clear acrylic that have the pipes diameters cut out as grooves 4mm deep are placed on the ends. The whole lot is glued together with 5 minute epoxy. A CNC was used to cut the acrylic but I guess you could just use hole saws the same diameter as the copper pipes to do the same thing.
Two small 2.5mm holes on one end cap are used to inject the gelled electrolyte in the cell with a bit of banging of the battery on the bench occasionally to get it all to pack in.
The resulting battery looks quite good and I hope to make bigger/longer versions soon as this will give me more surface area for a higher current draw and storage capacity.
If you want a picture of the cell Daniel, send me an email. The clear acrylic lets you see the gel go from white at the start to yellow with bits of orange/red from the bromine as it charges.
Cheers.
I should also say that I can’t dismantle the cell to check on dendrite formation unfortunately. But you know when the dendrites are causing problems when the battery charging voltage starts dipping erratically while charging. These pipe based batteries are only a new concept and were made to last longer than my other batteries where the electrode fails before any real performance can be obtained. So far with a gelled PEG400 battery I haven’t had a fail due to dendrites, but as you pointed out in a previous post my current mix only has around 0.8 mole of ZnBr2 in it so maybe there isn’t enough Zn to cause issues yet.
Thanks for your reply! With only 0.8M of ZnBr2 it is unlikely that the battery will be able to reach satisfactory energy densities (>20Wh/L). Have you ran tests with ZnBr2 at concentrations higher than 2M?
I just sent you an email! Hopefully you can send me a couple of pictures of your setup. The reason why you’re having problems discharging below 1.1V might have to do with copper oxidation. When you discharge your battery below 1.1V, you will start to get any exposed copper into solution, keeping your discharge voltage above 1.1V basically guarantees that exposed copper will not be oxidized as the battery is discharged.
Now that you mention it, I did see that in a paper somewhere! They used copper mesh at the top of the battery while still having a carbon type electrode on the bottom.
I have just bought a tester from https://www.aliexpress.com/item/4000606643595.html?spm=a2g0s.9042311.0.0.54284c4dfrTozz
In future I will have better results to share.
I sent you some photo’s as well 🙂
Cheers.
I would like a picture of this please.
Apparently I can’t post my email here.
Tim
Thanks for commenting. I have your email from your comment form. A picture of what exactly? Let me know!