During this past week I have been experimenting and thinking more about Zn-Br batteries and how a real practical battery would look like (how it would be built and what its characteristics would be like). Let’s imagine we have found a complexing agent that can be used in highly concentrated ZnBr2 solutions. What would a prototype battery look like and how much would it cost?
The first thing we need to consider is the geometry to build such a battery. Single-cell batteries for Zn-Br chemistry are impractical due to the limits that would impose on current density – and it’s not the 19th century – so the ideal battery would probably follow a configuration similar to modern lead-acid batteries, where multiple cells are put together to achieve better results. The simplest way to do this is to stack materials next to each other within a box, then flood the box with the desired electrolyte solution.
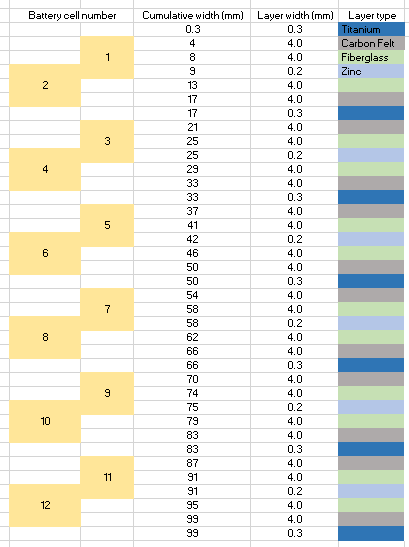
In a 101mm x 54mm x 44mm project box you could fit a volume of around 237mL. If we decide to use a very porous carbon felt electrode – which I have experience with – with titanium current collectors, glass fiber separators and zinc anodes, we would create a cell configuration like the one showed above. This would occupy the entire cell with either separator, current collector, anode or cathode material. Given that the materials used take little real volume, as they are either very porous or very thin, I’m going to assume the solution volume we will fit will be equal to 200mL, which is realistic given the characteristics of the materials.
If we use a 2M ZnBr2 solution, this would give a maximum theoretical energy of 40Wh. If the cells are all connected in parallel, this would give us a battery with a voltage of 1.85 at a capacity of 21.6 Ah. The battery would be charged at a constant current of around 2.85A, although depending on the actual kinetics this might need to go down to even 285mA. In the above design you actually have only 6 cells that are each equal to 2 normal cells connected in parallel – as they share a current collector in the cathode – so connecting these 6 in series would give you a voltage of 11.1V with an expected charging current of 475mA.
The main caveat of the above design is that it uses a 2M ZnBr2 solution, assuming we can find a complexing agent that forms an insoluble perbromide that can be in the initial formulation at a concentration equal to at least the same as the ZnBr2 then this should be no problem. After a lot of research about the solubility of perbromides and organic ammonium salts I believe this might be possible using trimethylphenylammonium bromide, but such a complexing agent has never been tried! The 200 mL of solution used here would use 90.07g of ZnBr2 and 86.45g of TMPB.
Note that this configuration would certainly not work without a complexing agent that precipitates the tribromide formed. Without it the bromine would pool at the bottom and discharge the cell – in a horizontal configuration – or just sink and discharge the cell in a vertical configuration.
| Cost (USD) | Item | URL |
| 3 | Project box | https://www.allelectronics.com/item/mb-132/abs-project-box-3.97-x-2.12-x-1.72/1.html |
| 45 | Carbon felt | https://www.ceramaterials.com/product/gfe-1-pan-graphite-felt/ |
| 10.99 | Fiberglass tissue | https://www.amazon.com/Multipurpose-Fiberglass-reinforcing-waterproofing-membranes/dp/B0719KWMJ7/ref=sr_1_5?dchild=1&keywords=fiberglass+paper&qid=1600038562&sr=8-5 |
| 11.49 | High purity Zn | https://www.amazon.com/99-9-Sheet-Plate-Metal-140x140mm/dp/B086FGDW83/ref=sr_1_5?dchild=1&keywords=Zn+sheet&qid=1600038696&sr=8-5 |
| 9.01 | Zinc Bromide | Price with shipping confirmed from Alibaba vendor |
| 19.99 | Titanium foil | https://www.amazon.com/0-3mm-200mm-300mm-Titanium-Purity/dp/B07G8YYPFV/ref=sr_1_2?dchild=1&keywords=titanium+foil&qid=1600040029&sr=8-2 |
| 18.85 | TMPB | Price with shipping confirmed from Alibaba vendor |
Using all the materials above, the cost of building such a prototype would be in the order of probably 120 USD. Probably around 200 USD after you add shipping for everything. In reality this cell is also unlikely to yield 40Wh and will most likely be in the vicinity of 20Wh if everything works as expected.
It is also important to note that an ABS project box like the one above is a risky first-choice, given that ABS can adversely react with elemental bromine, so a PTFE project box would – although much more expensive – be a safer choice for a prototype. By the time I build something like this, I hope I have already established that TMPB forms insoluble enough perbromide salts under my much more controlled Swagelok cell conditions.
Note that I am still far away from executing something like this! Currently I am even far away from testing a TMPB cell, but I wanted to write this blog post to condense all this theoretical research and serve as a referring point for me or others in the future.
Pretty much the design I had in mind as well. Will be interesting to see in practice if multiple series cells can share the same tank, or if the conductivity of the electrolyte will effectively parallel all plates that share any electrolyte contact. A 12v lead acid battery contains 6 separate electrolyte tanks for the cells, though each cell may have multiple parallel plates within its tank. A gel/AGM type design could probably accomplish a series stack, i.e. the classic voltaic pile, but tend to be considerably less robust than a simple flooded cell.
PVC (Type 1) is a cheap container option that is resistant to attack by halogens and is commonly used for pools containing chlorine and bromine. Stacking plate materials vertically in a piece of pipe and then compressing them gently with a screw clamp seems like a fairly simple option compared to PTFE boxes.
Is graphite felt as linked a poor enough conductor that it requires an expensive current collector? Titanium is almost 20% of your cost. I thought graphite was quite a bit more conductive than traditional carbon felts. Could it be more cost effective simply to keep stacking graphite plates in parallel until the desired current output is reached? Much like a low C-rate but high capacity lithium battery that can still deliver high currents. Of course it depends if the goal is current density, storage density or simply cost per kWh.
Graphene foam could probably combine the functionality of both the felt and the current collector. Like many people my first exposure to ZnBr cells was by stumbling across Robert Murray-Smith, who at one point attempts (successfully?) to synthesize graphene foam from sugar by following the procedure as described in https://www.nature.com/articles/ncomms3905 : “Three-dimensional strutted graphene grown by substrate-free sugar blowing for high-power-density supercapacitors.”
This material would appear to be an excellent potential cathode, with large surface area, good conductivity and near zero cost if manufactured on site. Pores are quite large, and “A High Performance Aqueous…” (2020) supports mesoscale pores being more effective than micropores. Unfortunately Robert seems to have quite poor focus and seems to have forgotten about the graphene foam long ago. He is always playing with something that interests him that day. You on the other hand are already getting somewhere with your battery project. Great to watch your progress, I wish I could work on this stuff but I farm and this time of year there is definitely no time for chemistry!
Thanks for your comment Alex!
The carbon felt I use has really good conductivity, at least as good as graphite as far as I can see. However the titanium current collector is going to improve the current collecting of the device because the conductivity of the felt is probably going to be somewhat impaired by the accumulation of the solid perbromide after the battery is charged. I want to keep it as simple as I can, but realistically accounting for a cathodic current collector seems reasonable as all practical Zn-Br devices I’ve seen use it, regardless of the conductivity of the carbon felt they use. Simple graphite plates won’t work though, because a high surface area is needed to be able to provide a significant current density, some pores are also needed to store the solid perbromide and keep it in close contact with the cathode after charge. Trying to use a bulk graphite electrode just causes a thin film of perbromide to form that isolates the electrode and prevents further charging.
I sadly don’t have the space or equipment to work on forming my own carbon materials so I will be experimenting with the ones I can get my hands on industrially for now. At this point I believe the biggest issue is getting the sequestering agent chemistry figured out properly (as you might see in my recent posts about TBABr not being very good). About Robert, I would certainly enjoy it if his videos had a lot of additional quantification, he always seems to talk about the practical aspects of things – especially dealing with construction – but I’ve never seen him thoroughly quantify his results. It’s a bit frustrating to watch someone build a battery and not provide a single charge/discharge curve or any other meaningful quantitative information.
Again I appreciate your comments, thanks again for your support!
My mistake, clearly structured graphite is necessary for the plates and when I said “plate” I was referring to the entire electrode. Should have written “graphite felt plate” instead. Looks like the material you are already using is a graphite felt rather than a simple welding blanket carbon felt due to high conductivity.
I like the idea of using EDTA to chelate the zinc in a cheap and heavy battery, but agree that getting a proper replication of the 2020 paper with TPABr is probably your best goal right now before getting too experimental. Interested to see your results with TMPhABr as well.
I have a much better environment here to produce carbon materials (as well as the requisite smoke and flame), and want to take a shot at them sometime myself. Originally I planned to replicate the Princeton “Minimal Architecture” cell with homemade graphitic foam, until the concept of sequestering the Br came along which is much better.
Note that the Princeton paper uses carbon for the anode as well – lighter than zinc and potentially helps avoid the dendrite issue as the carbon anode will not degrade and the zinc can be fully returned to solution via deep discharge.
Thanks for your reply Alex! I wrote an article about the current specific energy of my batteries and the direction I want to take them (https://chemisting.com/2020/09/25/zinc-bromine-batteries-what-would-be-realistically-required/), I would be interested in knowing what you think. Currently the zinc anode is a very heavy part of the battery but if I change to 0.02mm Zn foil, it is bound to become almost as light as the carbon electrodes. Also I’ve been using a variety of carbon electrodes, this is the felt I have used a bunch of times (https://www.ceramaterials.com/product/gfe-1-pan-graphite-felt/) but I have since used carbon cloths that are significantly lighter and seem to offer the same capacity (https://www.fuelcellearth.com/fuel-cell-products/carbon-cloth-variety-kit/). In particular the C4 carbon cloth is becoming a favorite.
Regarding the ZnEDTANa2, I think that a better solution for a low-cost implementation might be to use ZnSO4+NaBr, since these salts are significantly cheaper than ZnEDTANa2. The sequestering agents would be the most expensive part at this point, but both TPABr2 and TMPhABr can be obtained for relatively low costs in bulk (5 USD/Kg is a reasonable expectation for > 1 ton quantities for both).
About the Princeton use of carbon materials for the anode, this seems to become very problematic at larger scales when you’re forced to use lower cost carbon felts/cloths. The Princeton group actually made some attempts to scale up the battery, but they failed at the next level of scaling (see this thesis https://dataspace.princeton.edu/handle/88435/dsp01rj4307302). As far as I know the results of this thesis were never published in peer reviewed journals, probably because the results showed very important issues with the scaling of this type of battery. This is not very surprising given how big the self-discharge and energy loss problems are expected to be. It is also possible that the research was just stopped at this point because Daniel Steingart left Princeton for Columbia and Princeton holds the patent applications for this technology.
Stay tuned for my results using TMPhABr, which I will get sometime within the next two weeks. Thanks again for commenting!
Some ideas (warning some may be misguided and a a full rendering of the Dunning-Kruger effect)
1. It would seem the cheapest battery box would be made of HDPE
I searched for Zinc bromide HDPE and first link says,
“…Zinc bromide solution (UN 1760) is commonly supplied in liquid form as the following products: Zinc bromide , 73-77 (wt) % solution in water … Steel composite drum with an inner lining of HDPE…”
https://eif-wiki.feit.uts.edu.au/_media/technical:renewables:zinc_bromide.pdf
HDPE is everywhere. It’s empty milk cartoons and they can be melted down into whatever space you want in a standard oven. The forms could be sand casting.
My focus is on cheap. Ideally to make stationary batteries or even better batteries for to run motorhomes(RV’s). The idea being you put solar panels on the roof and if you park you have power and if you park for a while you make enough power to drive somewhere. With enough batteries you could park a few days then drive a day. Have diesel generators for back up power. Solar is going to get way cheaper. There’s a new solar cell that the factory is being built right now. Supposedly production starts this year. It uses combined Perovskite with regular solar cells to reach around 44% of the light falling on it into electricity. A RV takes around 20-30 hp to drive. With these sorts of efficiencies you could have enough power to drive on sunny days for free. With solar cheap enough the idea of having free mobile power will be affordable. It only needs super cheap batteries made from what could be called waste materials.
2. Cheapest electrodes. I may be in trouble intellectually here but activated carbon has been used for electrodes before. The simplest, cheapest way to make activated carbon is to bake it in a steel drum to drive off all the volatiles and what’s left is a very spongy open carbon matrix. (Hydrothermal Carbonisation)I can’t help but think if this was oriented correctly you might could get some capacitor effects also. Robert Murray-Smith has covered this and made batteries and super capacitors with this stuff. Here’s a link using tree waste to make activated carbon that looks interesting.
https://www.sciencedirect.com/science/article/abs/pii/S0045653514015070
There’s a LOT of these activated carbon creation links with tons of YouTube videos on taking steel barrels and making activated carbon.
People are doing this to make Terra preta while is a outstanding soil found in the Amazon originally made by the Indians there who disappeared from disease. There’s meters thick of this stuff in the jungle where vast villages used to be. Plants love it. They mine the stuff like fertilizer. VERY interesting. Here’s a link at random for activated charcoal electrodes
https://www.sciencedirect.com/science/article/abs/pii/S0045653514015070
Here’s a paper on extra preparation of tree material used in making the activated charcoal.
https://www.hindawi.com/journals/jchem/2013/489670/
Things that I think about. Is it necessary to use the above paper to clean the wood before cooking?? Could it be that the minerals in the wood are not a problem?? They might could even help.
A good wood for this would be mass produced pine. They grow this stuff super fast in plantations so it will have large pores. Pine boards or two X fours for prototypes are readily available and would be good for experiments.
Some separator videos
Solid electrolyte for supercapacitors
https://www.youtube.com/watch?v=Ogpeaklho_M
Acetate
https://www.youtube.com/watch?v=-g9MQ8e93Yw
Robert Murray-Smith has a video on making separators from cheap silicon you get at hardware stores. Might save you a lot of money and the pores are chemically formed so, I believe, that could be very small uniform and controlled. It even uses the cheapest silicon.(I can’t find this video online. I know it exists. I didn’t save it. I suspect it on his members only channel).
Activated carbon, I think, from sugar
https://www.youtube.com/watch?v=LZMwAzaeGA8
Carbon Ink With Higher Conductivity Than Metal
https://www.youtube.com/results?search_query=Robert+Murray-Smith+Carbon+Ink+With+Higher+Conductivity+Than+Metal
There’s a wealth of stuff on Robert Murray-Smith’s channel but it’s difficult to find because he jumps around so much.
It seems that if you could get a little performance off of traditional battery operation and add in a little super-capacitor with it you could get good all over performance like Robert is getting. If you could get lower end li-ion performance I suspect that would be plenty for my uses and ok for cars and trucks if you removed the motor.
Hi Sam,
Thanks for sharing you thoughts! A few things on your suggestions:
1. HDPE won’t work because it reacts with elemental bromine and perbromides produces in the reactions that happen within the battery, so sadly it can’t be HDPE.
2. Electrode fabrication is sadly not that simple, the activated carbons you make in setups like that are low conductivity. Making high conductivity, high surface area carbon materials that have the proper structure is not trivial. However there are already companies that produce highly graphitic felts that can be used for battery constructions at fairly low cost levels, see the GFE-1 cathode material I use.
3. Separator materials need to comply with the requirements of the battery chemistry, elemental bromine and perbromides are very reactive and only a handful of materials can really withstand this chemical environment. When you have new electrolyte that is just Zinc Bromide it is fairly benign but once you charge the battery you start generating some very unfriendly chemistry for most materials.
4. Bear in mind that although I really appreciate Robert Smith’s efforts to get people excited and into their own experiments, he rarely characterizes any of his experiments properly – at the standards you would expect to draw anything quantitatively useful – so it is really hard to evaluate whether anything he makes is really good or bad. For example his Zinc Bromide batteries have no charge/discharge curves measured – the most basic measurement you would expect to say something useful about any battery – and his material and geometry choices imply he should be getting really bad energy efficiencies, so although great for getting people into things, I would take anything he posts with a grain of salt. What he usually describes as “great results” should not be taken quantitatively as such unless they are put under the usual scrutiny anyone making such claims would face. Robert is a guy who wants to show people things and he does a great job at it.
You have the excitement of someone who has read a lot, watched a lot of views but has probably not done a lot of actual experimentation on the matter. I would encourage you to start doing some quantitative experiments so that you can get a sense of the complexities and the problems of these or other batteries. When just reading papers or watching videos a lot of problems are not apparent but actual experimentation will allow you to get a sense of why this issue is so difficult and why there’s really no massive adoption of Zn-Br batteries despite decades of research on the matter. Thanks again for posting!
Hopes…dashed! I was really counting on the HDPE because it’s everywhere. And you’ve pegged me completely I read a fairly good deal about this but haven’t built much. Robert’s stuff is great but your criticisms are fair. He is trying to make a living at this stuff so….he’s a bit vague at times.
We really need a battery with say the Wh\kg of a lower Li-ion battery that is super dirt cheap to make yourself. That would be for me the sweet spot. Of course more is always better.
Another idea for containers is glazed pottery with wood and cardboard as container backing.
Anyways I’m not to sure how much of this I will actually build unless I can find instructions to do so. My chemistry skills are very weak. I took Ch101 and 102 but it was like three decades ago. Sure I did get the basics but as you know chemistry is a whole massive amount of little tricks that people have built up over hundreds of years. Difficult to pick up and some of it very much closer to art than science. I will keep on looking about though and maybe something will come up.
Forgot to add. I think with the increases in the ability to manufacture graphene and lowering cost of carbon fiber and carbon nanotubes that flywheels could make a serious run for the money compared to any chemical battery. Batteries are such a huge pain in the ass with the complexity involved and so many variables. Not to mention how exactly can you prove this stuff will work over decades just by charging and discharging in a lab? Lots of risk.
Flywheels are different . It’s fairly easy to determine the strength of a structure and how it will degrade compared to any chemical battery. If the materials get cheap enough I suspect to see movement in that direction. Depending on the level of stress used these things could easily last a lifetime.
I don’t know if this will work(formatting) but this is a few sets of data I keep in a text file that I scrounged from all over.
Theoretical potential for flywheels with contemporary and future materials
Type Cost
$ per Wh Wh/kg Joules/kg Wh/liter
Lead-acid $0.17 41 146,000 100
Alkaline long-life $0.19 110 400,000 320
Carbon-zinc $0.31 36 130,000 92
NiMH $0.99 95 340,000 300
NiCad $1.50 39 140,000 140
Lithium-ion $0.47 128 460,000 230
Lithium-ion tesla present 250
Lithium-ion tesla dry 300 path to 500
Material Tensile strength Density Spec.strength Breaking length Source Rotor energy density
(MPa) (g/cm³) (kN·m/kg or kYuri) (km) (Wh/kg)
Maraging steel 900 8.0 ET 24
Titanium alloy (Beta C) 1250 4.81 260 26.5 ET 31
Composite carbon fiber – 40% epoxy 1550 1.550 ET 52
Basalt fiber 4840 2.70 1790 183 wiki 213-247.65 guess
Multi-walled carbon nanotubes(low end)10,000 1.750 5714 (T.P.F.C.F.M.) 793
Boron nitride nanotube 33000 2.62 12,595
Multi-walled carbon Nnanotubes(high end)60,000 1.750 34,285 (T.P.F.C.F.M.) 4761
Single wall carbon nanotube(low end) 50,000 1.300 38,461 (T.P.F.C.F.M.) 5341
Carbon nanotube (see note below) 62000 0.037–1.34 46268–N/A 4716–N/A wiki
Colossal carbon tube 6900 0.116 59483 6066 wiki
Graphene 130000 1.0 130,000 wiki/Ultimate_tensile_strength 17,944
Graphene (Wh/kg) calculated from multiplying the Spec.strength(652) of E-glass (glass fiber) to get Wh/kg)
Single wall carbon nanotube(high end) 500,000 1.300 384,615 (T.P.F.C.F.M.) 53,418
130000
Fundamental limit 9×1013 9.2×1012 wiki
Hmm I think this may be a mess (my apologies if it didn’t come out but I can’t really tell until I post it)but maybe it will work. I want to point out the Wh/kg based of Spec.strength of a few
graphene is 17,944 Wh/kg
Single wall carbon nanotube(low end) 5341 Wh/kg
Multi-walled carbon nanotubes(low end) 793 Wh/kg
Composite carbon fiber – 40% epoxy 52 Wh/kg
Basalt fiber 213-247.65 interpolation Wh/kg
Now these numbers are high, when you add supporting structure and other stuff but some of the higher ones have a LOT of room to work with.
Basalt fiber is interesting. Say you lose half the Wh/kg you still have a good battery that could used in a car or truck that recharges as fast as you can feed it power. Basalt fiber is being used to replace steel rebar in concrete at slightly higher prices so cost would be low. An integrated factory where this stuff is automated could make very cheap batteries.
Greetings!
Have you made any progress in your experiments to make a practical Zinc Bromide battery for stationary use? I am an off- gridder looking for information to build one. I am a layman so I do not understand chemistry enough to experiment on my own.
Thanks for writing Cari! After a lot of experiments using Zn-Br chemistry, I decided against more experimentation due to the magnitude of the challenges required to make a reliable and robust Zn-Br battery. I would advise against using this if you want to create a DIY battery. Please read this forum post for more information about why I stopped working on them (https://diysolarforum.com/threads/my-adventures-building-a-zinc-bromine-battery.11910/page-14#post-290510).