In order to study the chemistry of Zinc-Bromine batteries I have been using a swagelok cell that I bought from China that has a central Teflon body with stainless steel electrodes. This has been problematic due to the reactivity of these electrodes with the elemental bromine and tribromide salts produced in the battery, requiring the use of some “improvisation” in order to make the batteries work.
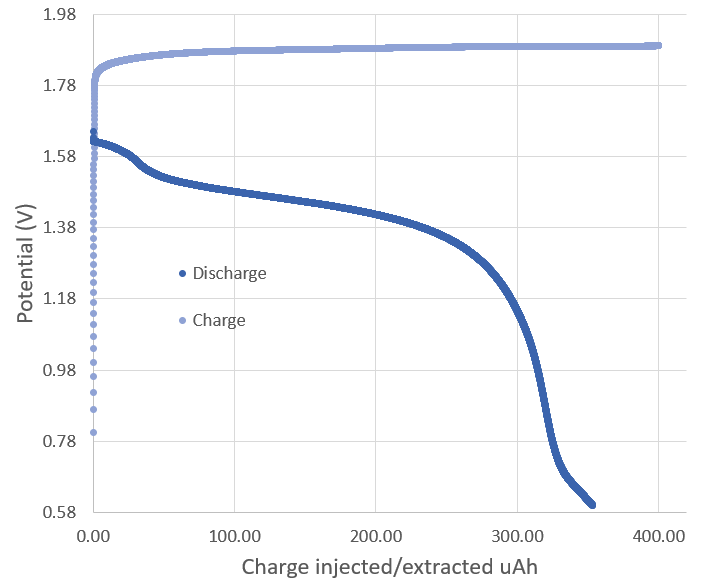
To be able to generate the necessary chemical reactions without interference from the stainless steel I have coated the electrodes with some conductive HDPE I have, which has a relatively high volume resistance of around 10K ohm. The charge/discharge curve above shows you the type of measurements I have been able to achieve with this setup, with the above curve having a Coulombic efficiency of 88%.
This is lower than what I could achieve with the copper anode I was using previously (which gave me around 96%), but note how the charging voltage is lower and the discharge voltage higher, meaning that the overall energy efficiency is significantly better (around double). This is however not because of the zinc anode, but because with the conductive HDPE now covering both electrodes, I have now been able to tighten the cell more and achieve a lower overall internal resistance.
However the still relative low energy efficiency and voltage drop when going from charge to discharge are still pointing to significant sources of efficiency loss, possibly from the 10K ohm resistance that the conductive HDPE is giving. The weird shapes at the beginning and end of the discharge curve are also pointing to more than one chemical reaction happening, probably because the electrodes are still somehow interfering with the chemistry (maybe some micro holes in the conductive HDPE at points of stress are exposing the electrodes underneath). For this reason I have decided to get graphite electrodes for the Swagelok cell, which I will build from these 0.5 inch diameter graphite rods I found.
I am also going to change the current carbon felt cathode for carbon paper electrodes – which are on the way – but I will refrain from using the paper until I can perform some tests using actual graphite electrodes that are guaranteed to be free of any pesky side reactions, with way less resistance than this conductive HDPE.
Hi, I just found your blog and work. I’ve been following ZnBr development with some interest as an electrician who works with renewables, and also studied chemistry in university. It’s great to see a hobbyist trying to properly quantify the performance of these fairly simple cells. I hope to have time to work on this chemistry as well and maybe build a prototype myself in the winter.
My personal interest is more towards a tank cell for bulk off-grid storage, similar to that described in the paper “Minimal architecture zinc-bromine battery for low cost electrochemical energy storage” rather than a pouch or cylindrical cell. It appears from previous experiments that exposure of the components to air is not detrimental to them, and a simple plate stack of carbon/separator/zinc in a tank of electrolyte would be easier to experiment with as well as more scalable and simpler to manufacture if successful. With the complexing agent precipitating the bromine into the pores of the carbon, a vertical plate arrangement might be feasible making the build very simple. The hazard of bromine escaping would be significantly diminished by it being in solid form. Opinions?
In comparison the fussy Swagelok cell seems like more trouble than it’s worth, honestly. The 10k resistance of the HDPE almost certainly is throwing off your results.
The data presented in “A High-Performance Aqueous Zinc-Bromine Static Battery” is potentially game changing for the off-grid industry if you succeed in replicating it in a scalable fashion. Interested to follow your progress.
Thanks for your comment Alex! I appreciate your support!
About the vertical cell stack, sure, I don’t see why you couldn’t build a large battery where you have stacks of carbon/separator/zinc. If the carbon used is porous enough all the TBAB tribromide will be trapped within it, so it’s unlikely to interfere or migrate significantly between the separators. That’s likely what I want to build at some point but I want to be really sure about the chemistry and materials in a small scale before going any bigger. I want to use as little material as possible while learning about the chemistry of the cells.
The Swagelok cell is definitely not something you would use for any practical purpose, just a way to study the chemistry. Of course, you’re right about the 10K resistance being a huge problem at the moment, but this will all be eliminated once I change the current electrodes for graphite rods within my Swagelok cell. Stay in tune for some of these results in the coming weeks.
Thanks again for your comments! Greatly appreciate any input I can get!