Zinc bromide flow batteries have been researched very extensively during the past 30 years. There are many advantages to this chemistry, very high potential (~1.8V), high efficiencies, symmetric electrolyte and low reagent costs. Nonetheless, the disadvantages are also huge: zinc dendrites, hydrogen evolution, bromine corrosion, etc. Despite all the development, a lot of these disadvantages remain insurmountable.
A recent nature paper has disrupted the field by using sulfamate ions as a bromine scavenger. Unlike previously used complexing agents that sequestered Bromine as reactive Br3- species, the new scavenging method sequesters Bromine as an N-bromosulfamate, which is stable in solution in the timescales necessary for energy storage. Furthermore, the N-bromosulfamate is chemically much milder than elemental Br2 or Br3-, making the use of cheaper gasketing materials possible and preventing a lot of issues associated with the high reactivity of elemental bromine species.
I have been very excited by these findings and have ordered some sodium sulfamate to test this chemistry myself in our FBRC development kit. However, the development of this technology is likely not open source and it is very likely that the people involved with it want to patent it and lock down the technology. This made me think about potential alternatives that could be used outside of the sulfamate family that could also exploit the mechanism of Br storage in N-Br bonds. Such a technology might be outside the scope of their original paper and therefore be exempt from intellectual property registration.
Thinking about the stability of N-Br compounds (usually not stable at all), I immediately think about NBS and analogous chemical compounds. These are very stable reagents that are routinely used in chemical synthesis, although their aqueous solubility is very low and therefore not useful in the creation of a ZnBr2 aqueous battery.
With that said, nicotinamide (vitamin B3) is a very water soluble and readily available material that also forms a stable N-Br compound in mildly acidic conditions. This 2007 paper describes how N-bromonicotinamide can be created using elemental bromine. While N-bromine compounds from amines like this would often go through a Hoffman rearrangement to yield the corresponding amine, this doesn’t happen under mildly acidic condition in the case of nicotinamide. In fact, the 2007 paper mentions that a concentrated solution of this N-Br compound was stored for months without degradation. The solubility of nicotinamide is also very high (5-6M), compared to sodium sulfamate’s solubility limit of 1.3M at 25C.
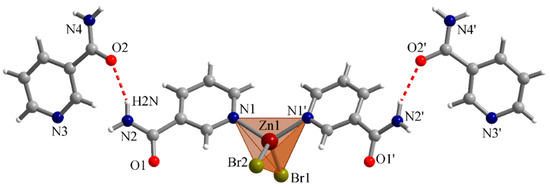
Furthermore, nicotinamide forms mono and dimeric complexes with Zn atoms through the nitrogen in their pyridine ring, which makes this nitrogen unavailable for potential deactivation with direct bromination of this N to yield the corresponding quaternary nitrogen salt (irreversible and undesirable in the context of a battery).
Given that vitamin B3 is very soluble, very low cost, already produced industrially, has a stable amide N-Br compound and is unlikely to undergo Hoffman rearrangement or similar decomposition modes, it is a great candidate to serve as a Br2 scavenger in a ZnBr2 battery. I am going to buy some vitamin B3 to test this idea out. Stay tuned for some tests of this and the sodium sulfamate chemistry.