Trying to improve on Zinc-Bromine batteries, it seems that the use of non-aqueous solvents in order to better sequester the bromine would be ideal since successfully sequestering the bromine in a static battery configuration is hard, even when ammonium salts are used as sequestering agents. This is because the salts are largely insoluble in ZnBr2 solutions, so the sequestering agent needs to be included as a solid, which can only be done in very limited amounts without strongly degrading battery performance. This is what I have tried to do with TMPhABr saturated GFE-1 cathodes, but the amount of Bromine I can sequester before it leaks out into the rest of the cell is quite limited. This is evident when I take apart fully charged cells and the entire cell is tinted with a strong orange color.
Ideally we would want to have a phase that is separate from the aqueous ZnBr2 phase but that has a higher affinity for bromine, is able to dissolve a significant amount of ZnBr2 (>1.5M) and is non-toxic. Propionitrile has been shown to be a decent candidate to achieve this, see here, but it is not an ideal candidate for a DIY solution because it is hard to get in most cases and can be a significant hazard for human health. It is catalogued as a dangerous chemical for transport in the US and EU and its use therefore poses a lot of additional restrictions and problems.
Most polar solvents that can dissolve ZnBr2 to some extent and are electrochemically stable – meaning they won’t get oxidized at the cathode – are sadly also miscible with water. This means that they don’t form a separate phase, which is an absolute requirement. I tried using propylene carbonate mixed with tetrabutylammonium bromide (TBABr) – which does not mix with ZnBr2 solutions – but this was a complete failure due to the fact that the bromine and perbromides formed actually prefer the ZnBr2 phase to this organic phase, even at lower concentrations of TBABr.
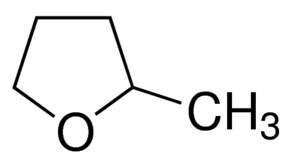
I then continued my search for the “perfect solvent” for this, reviewing a lot of the literature on non-aqueous solvents in batteries and on “green chemistry” to find the solvent with the perfect polarity, ability to dissolve Zn-Br, affinity for Bromine, immiscibility with water and low toxicity. I think I might have found a solution in the form of 2-methyltetrahydrofuran or Me-THF. This solvent is a highly polar ether, it can dissolve high amounts of ZnBr2, it has a high affinity for elemental Br, it’s significantly less toxic than propionitrile and, alike propionitrile, it does not mix with water or ZnBr2 solutions. It is also electrochemically stable and is also safe enough that it’s available for purchase on ebay/amazon, so I decided to buy some of it to do some basic experiments and see if this solvent finally provides the answer we have been looking for,
Interestingly the density of Me-THF is quite low – around 80-90% that of water – so it will naturally rest on top of it. This means that it is naturally suited for an inverted cell configuration (cathode on top, anode on the bottom), which is what we want (see here). I couldn’t find what the partition coefficient with ZnBr2 solutions in water is, but the hope is that we should be able to get enough ZnBr2 in the ether to have a successful battery starting with a 3M solution of ZnBr2 in water. Batteries with Me-THF likely won’t require the use of any ammonium salts – as in the propionitrile paper mentioned above – since the affinity of the Br for Me-THF is high enough to keep it away from the aqueous phase.
Alike with PC, it is hard to know what to expect, so I would advice waiting for some of my results before going out to buy this solvent. Who knows how it will work in practice!
Hello, I just found your research today and have been reading as much as possible.
Are you still continuing your work with these batteries? I would love to someday be able to build a large system utilizing easy to build and affordable batteries.
I am waiting for your research to avoid any mistakes I could make.
I do hope you are continuing your research and I would love to contribute any help I can.
Are you still working on this!?
Very interested in your progress!
This is a great idea that may solve many problems.
Can u tell us the result?
If u haven’t finish it, I may take this into my plan
I tried it, but Me-THF just smells too much and I couldn’t work with it at my house. I did some tests, which were not very encouraging, but definitely did not try it extensively.