In my first article about zinc-bromine batteries I discussed why these batteries are gaining interest and how some recent articles point to their potential use as reliable and cheap batteries, especially for large scale applications. After building my own DIY potentiostat/galvanostat, I wanted to use this technology to characterize home-made zinc-bromine batteries and experiment with their chemistry.
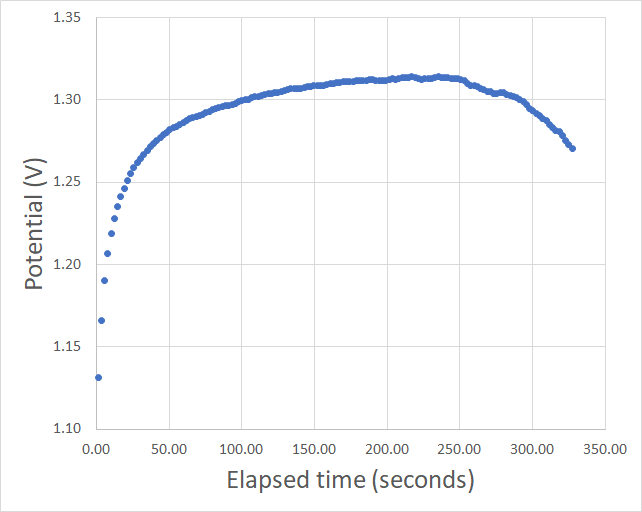
My previous article also mentioned some of my first attempts at building these batteries, which were mostly failed attempts due to the complexity of the battery builds. Even though I was able charge the batteries a little bit – and obtained relatively high Coulombic efficiencies when injecting a small amount of charge – I was never able to sustain potential values close to the expected 1.6-1.8V of the zinc bromine system. Always topping up at around 1.3-1.35V as shown in the image above, when trying to inject charges at 1mA/cm^2.
A huge problem of my first set of designs was a complete inability to adequately reproduce my batteries. The electrode construction was very complicated and every battery I tried had slightly different geometry and different amounts of electrolyte within their construction. In order to standardize the study I decided to change to a Swagelok cell construction (which I bought from China here). I bought a cell and got it delivered to the US within one week.

Although the Swagelok were supposed to make things easier, I started to face issues with the electrode material of the cells being reactive towards the bromine generated within the battery charging process. In my initial attempts using a carbon felt electrodes and a fiberglass separator, the stainless steel electrodes in the cell – which are inevitably exposed to the solution – were getting corroded away by the generated bromine and tribromine salts.
I was finally able to surmount these issues by covering the Swagelok cell electrode pieces with conductive HDPE, basically by wrapping the electrode with it and then inserting it within the Swagelok cell. Using this method I was able to produce my first successful Zn-Br cell using a tetrabutylammonium bromide (TBAB)/ZnBr2 solution (0.25 and 0.5M respectively) , a copper electrode for zinc reduction a fiber-glass separator and a carbon felt electrode for the tribromide depositing.
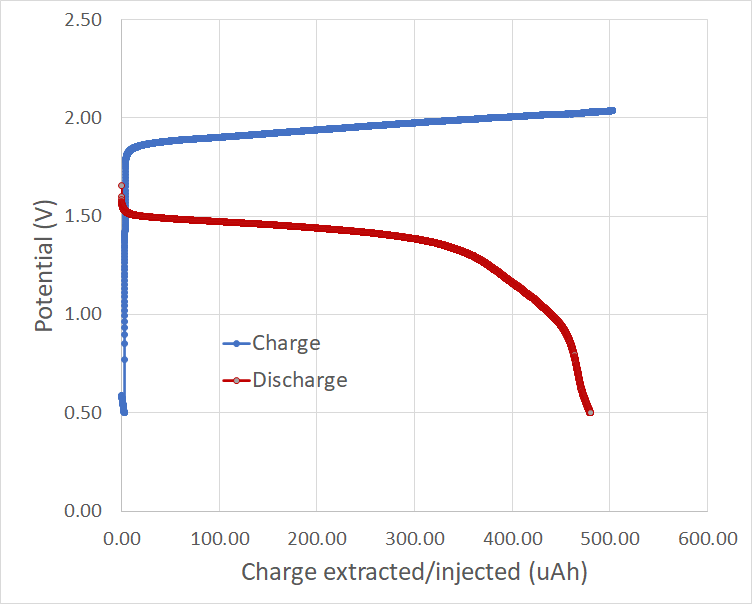
The image above shows you my first successful charge/discharge curve. To the best of my knowledge, this is the only example available online for experimental data of a TBAB/ZnBr2 cell. The Coulombic efficiency of the above cell was 96%, which is great considering this is the first successful one I have built. The cell used around 80-100uL of solution and 4 layers of fiber-glass separator (see my previous post for links to these materials).
I am still facing some issues related with the cutting of the separator/electrode materials to place within the cell (I have bought a 0.5 inch cutter which should make this way easier) and I am also going to try using a zinc electrode for the zinc plating, which should make things easier. I also want to see if I can get a better non-reactive conductive coating for the cell electrodes, since the conductive HDPE I am using has a quite significant resistance. Things are looking up though!